4454
In search of the right solution for Ex-Vivo Diffusion Weighted Imaging
Shijia Teo1, Jochen Leupold1, Juergen Hennig1, Marco Reisert1, and Valerij G. Kiselev1
1Dept. of Radiology, Medical Physics, Medical Center - University of Freiburg, Faculty of Medicine, University of Freiburg, Freiburg, Germany
1Dept. of Radiology, Medical Physics, Medical Center - University of Freiburg, Faculty of Medicine, University of Freiburg, Freiburg, Germany
Synopsis
Utilising the right solution in immersing an ex-vivo sample is the first and yet important step in optimising dWI, while preserving the biological and structural integrity of the sample for effective and meaningful repeated scans. In so doing, using 1%PFA, while toxic in preparation, has many other benefits in optimizing diffusion-weighted imaging (dWI). For a safer handling option, PBS + 0.005%NaN3 is also a viable alternative.
Introduction
Human ex-vivo tissues are gaining traction in validating in-vivo microstructural MRI. While tissue treatment for traditional histology is well established, it is blind to NMR parameters such as diffusion coefficients, T1 and T2, which are central for many in-vivo techniques. But they are unstable as tissue degradation decreases apparent diffusion coefficient (ADC) and fractional anisotropy (FA), amongst other properties2,3. Diffusion ex-vivo largely depends on the solution used to immerse samples and a myriad of solutions is reportedly used without clear rationales. Ostensibly the selection of the appropriate solution is tailored for the specific parameters of individual experiments, however it is crucial not to ignore aforementioned scan properties to prevent compromising scan image quality. The aim of this study is henceforth to investigate four most popular solutions that can promote high quality diffusion-weighted imaging (dWI) while also taking into consideration bactericidal properties and safety levels of these solutions for repeated scans.Materials and Methods
Ex-vivo brain preparationThe ex-vivo brain was obtained from the Pathology Department, University Medical Centre Freiburg. It was female, over 80 years old with no known neurological medical history or pathology. The body was fixed with formalin from the femoral artery and the brain was extracted upon complete perfusion.
Preparation of sample
Brain pieces were sectioned out for high-resolution scanning. Dimensions of the pieces are 2.3x1.5x5cm3. Brain pieces were incubated in respective solutions for at least 24 hours before scanning. The investigated solutions are listed in Table 1.
MRI acquisition
Ex vivo scans were made with 9.4T Bruker scanner with a bore diameter of 200 mm. T1 relaxometry rapid acquisition with rapid enhancement (RARE) scanning parameters were: 6 TRs from 200ms-10000ms, TE=7ms with resolution of 0.1x0.1mm.T2 relaxometry multi-slice multi-echo (MSME) scanning parameters were: 30TEs from 8-240ms, TR=2000ms with resolution of 0.4x0.4mm, lower resolution necessary to avoid diffusion shine-through on the signal decay over echo time6. Both T1 and T2 were performed with FOV = 26x26mm and slice thickness = 1mm. Diffusion scanning parameters were: TE=40.22 ms, isotropic resolution of 0.18 mm, b value=2000 m/s2. Spectroscopy scanning parameters were: Point RESolved Spectroscopy (PRESS) sequence: Bandwidth in ppm = 12.49ppm, spectral resolution = 1.22 Hz/points, acquired bandwidth=5000 Hz, averages = 160, TE=20ms, TR=4000ms, voxel size=3x3x3mm
Analysis
T1 and T2 relaxivities were calculated using Bruker Image Sequence Analysis tools with mean and standard deviation measured using voxel size of 7.5x10-2cm2 over WM, GM and surrounding solution. MD values averaged over regions of interest (ROI)s as highlighted in Figure 2 were calculated using NORA, a medical imaging platform: [http://www.nora-imaging.com/].
Results and Discussion
Four often used solutions were selected for this investigation from a general literature overview as well as informal advice from pathology department:A. 2 Phenoxyethanol is typically used to preserve ex-vivo tissue and is a safe preservative which washes out extra formaldehyde, and an efficient bactericide at low concentrations4;
B. Phosphate buffered saline (PBS) is a commonly used in handling biological tissue samples, with NaN3 at 0.005% concentration to facilitate antimicrobial environment for long term storage;
C. 1%paraformaldehyde (PFA) is a common fixative and bactericidal solution for biological tissues;
D. Galden(perfluoropolyether solution) is sometimes used in immersing samples for dWI, purportedly for its inert properties in matching susceptibility1.
Shorter T1 times would be more advantageous in boosting signal intensity and augmenting spatial resolution4, thereby making 1% PFA (Figure 1a-c, Table 1) solution desirable for dWI. As a signal free solution, sample immersed in Galden has nonetheless displayed the presence of signal (albeit very low) in T1 and T2 (Figure 1d & e), which is very likely derived from residual water leaching out of the sample into its surrounding solution, which is supported by presence of water peak in spectroscopy (data not shown). This finding can be useful in studies aiming at singling out individual cellular compartments. PBS + 0.005%NaN3, has the longest T2 relaxation time (Figure 1b, d and Table 1). Long T2 time is beneficial in dWI in allowing longer TE, thereby conceding sufficient diffusion time and contrast without sacrificing signal owing to T2 relaxation time4.
Another parameter critical in this consideration is MD, in which 1%PFA displayed the highest value within the sample. This strong diffusivity property of 1%PFA is highly valued in dWI, which better highlights and differentiates highly organised microstructures from disorganised ones.
In Figure 3, the FA maps of WM in brain tissue sample further the value of 1% PFA in dWI, in that the tracts can be discerned in anisotropically diversive directions (Figure 3c). This is in contrast to FA maps of sample in 2 Phenoxyethanol and Galden, whereby tracts appeared more isotropic (Figure 3a & d).
2 phenoxyethanol and Galden are safe solutions to prepare and handle, whereas preparing NaN3 in PBS can be harmful to the handler and environment, though not as dangerous as 1%PFA. Despite the toxic nature of 1%PFA, it is effectively bacteriocidic and preserves ex-vivo tissue well even in room temperature5, thereby making it a desirable solution of choice for dWI. PBS + 0.005%NaN3 is also a viable option in producing optimal dWI, though tissue should be stored at 4°C when not used for scanning to optimise preservation.
Acknowledgements
This study was supported in part by the Excellence Initiative of the German Research Foundation (GSC-4, Spemann Graduate School) and logistical support with regards to the post mortem brain by the members of the Stereotaxic Neurosurgerical department of the University Medical Center.References
- Wu, D., Martin, L., Northington, F., & Zhang, J. (2014). Oscillating gradient diffusion MRI reveals unique microstructural information in normal and hypoxia-ischemia injured mouse brains. Magnetic Resonance In Medicine, 72(5), 1366-1374. doi: 10.1002/mrm.25441
- Miller, K., McNab, J., Jbabdi, S., & Douaud, G. (2012). Diffusion tractography of post-mortem human brains: Optimization and comparison of spin echo and steady-state free precession techniques. Neuroimage, 59(3), 2284-2297. doi: 10.1016/j.neuroimage.2011.09.054
- Miller, K., Stagg, C., Douaud, G., Jbabdi, S., Smith, S., & Behrens, T. et al. (2011). Diffusion imaging of whole, post-mortem human brains on a clinical MRI scanner. Neuroimage, 57(1), 167-181. doi: 10.1016/j.neuroimage.2011.03.070
- Barrett, R., Cash, D., Simmons, C., Wood, T., Vernon, A., Catani, M., & Dell'Acqua, F. (2018). Optimal tissue preparation for ex vivo preclinical imaging. In ISMRM. Paris. Retrieved from http://indexsmart.mirasmart.com/ISMRM2018/PDFfiles/3240.html
- Brenner, E. (2014). Human body preservation - old and new techniques. Journal Of Anatomy, 224(3), 316-344. doi: 10.1111/joa.12160
- Oakden, W., & Stanisz, G. (2014). Effects of diffusion on high-resolution quantitativeT2MRI. NMR In Biomedicine, 27(6), 672-680. doi: 10.1002/nbm.3104
Figures
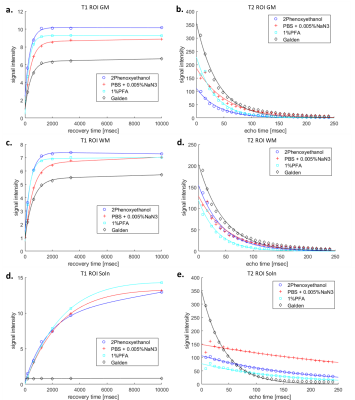
Figure 1. T1 and T2 relaxivities of 4 different solutions in which the samples were stored and scanned in a), b) grey matter (GM), c), d) white matter (WM) of the samples point to 1%PFA and 2 Phenoxyethanol as desirable solutions with short T1 and long T2 time respectively. For comparison purposes, T1 and T2 maps of the e), f) solution outside the tissue were also scanned.
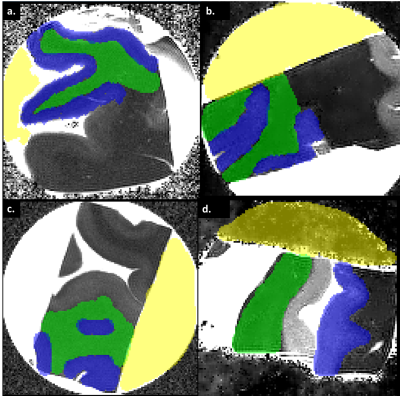
Figure 2. Mean diffusivity (MD) maps of braincubes in a) 2 Phenoxyethanol, b) PBS + 0.005%NaN3, c) 1%PFA, d) Galden. Areas coloured green is in WM, blue is in GM and yellow is in the solution surrounding the tissue. Corresponding MD values reflected in the following table indicates 1%PFA solution to have high MD, thereby making it a desirable solution for ex-vivo dWI.
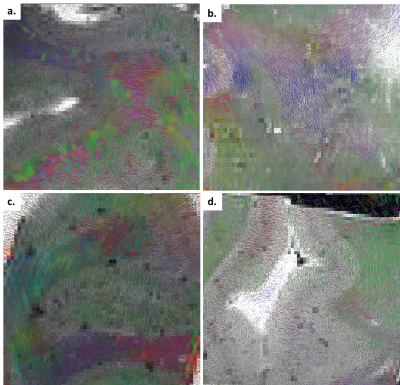
Figure 3. Coloured-fractional anisotropy (FA) superimposed on Bo anatomical scan image of a) 2 Phenoxyethanol, b) PBS + 0.005%NaN3, c) 1%PFA, d) Galden indicates that braincube in 1%PFA solution display meaningful anisotropic fibre tracts within the WM compared to the WM immersed in other solutions.
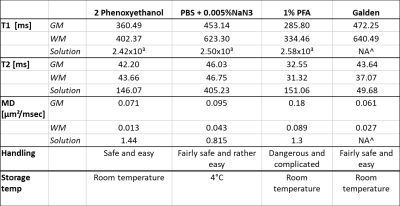
Table 1. Summary of varying solutions with their corresponding T1 and T2 relaxation times, as well as their respective MD and level of difficulty in preparation seem to indicate that 1%PFA solution may be beneficial in contributing to optimal diffusion weighted imaging (dWI), though not wholly conclusive.^NA: Non-Accessible data, as the value was too low to be registered under the Bruker Image Sequence Analysis software.