4447
Microstructural brain abnormalities and reorganization of early-blind adolescents: a voxel-based diffusion kurtosis imaging study1Radiology, Shenzhen Mental Health Center/Shenzhen Kangning Hospital, Shenzhen, China, 2Shenzhen Mental Health Center/Shenzhen Kangning Hospital, Shenzhen, China, 3GE Healthcare, Beijing, China, 4The First Affiliated Hospital of Jinan University, Guangzhou, China
Synopsis
An important focus of blind brain research, especially the early-blind brain, is how to identify the specific neural plasticity patterns. Neuroimaging studies, particularly the diffusion MRI, are powerful probes for characterizing the microstructural changes in human brain. Additionally, previous study indicated that the Diffusion Kurtosis Imaging (DKI) is an advanced diffusion model without the assumption of Gaussian distribution. Taken together, it is feasible to utilize the DKI to investigate the structural neuroplasticity in early-blind brain. Our results demonstrated that the neural reorganization and compensatory development process induced by visual deprivation are coexisted in early-blind adolescents. Furthermore, the diffusion kurtosis metrics are more sensitive to detect the pathology and development related brain regions than diffusion tensor metrics.
Introduction
Blind people is a natural bio-model for the investigation of the neural mechanism in populations with visual deprivation. An important focus of blind brain research, especially the early-blind brain, is the identification of neural plasticity patterns. Neuroimaging studies, particularly the diffusion-based MRI methods, are powerful probes for characterizing the effects of disease and neural development on tissue microstructure. Compared to conventional diffusion tensor imaging (DTI) based on Gaussian diffusion model, diffusion kurtosis imaging (DKI) is expanded towards quantification of non-Gaussian water diffusion, which is closer to the physiological state and more sensitive in detecting pathology in the grey matter as well as in white matter.1Taken together, it is feasible to utilize the DKI to investigate the microstructural changes and neuroplasticity of brain induced by early visual deprivation. To test our hypothesis, in current study, 23 early-blind adolescents (EBAs) and 20 age- and gender-matched normal-sighted controls (NSCs) were applied.
Method
DKI and 3D high-resolution T1-weighted structural image of 23 EBAs (15 males and 8 females; age range 11-18, 14.80±2.07 years) and 20 age- and gender-matched NSCs (9 males and 11 females; age range 11-19, 14.46±2.62 years) were acquired. By using the FMRIB Software Library (http://www.fmrib.ox.ac.uk/fsl/), the DKI data were first preprocessed for motion correction, eddy current correction, and b0 image generation of each subject. Then, DKI parameter maps, including four tensor maps, i.e., axial diffusivity (AD), radial diffusivity (RD), mean diffusivity (MD) and fractional anisotropy (FA), and four specific kurtosis maps, i.e., axial kurtosis (AK), radial kurtosis (RK), mean kurtosis (MK) and fractional anisotropy of kurtosis (FAK), were calculated from Diffusion Kurtosis Estimator software (https://www.nitrc.org/projects/dke/). After that, T1 anatomical image of each subject was first registered to b0 image using SPM12 (http://www.fil.ion.ucl.ac.uk/spm) with a six degree-of-freedom transform. Then the co-registered T1 images were non-linearly normalized from individual space to MNI 2 mm space. Last, all the DKI related quantitative maps were transformed to the MNI space. To quantity the statistically significant different brain regions between the two groups, two-sample t-test were performed at the whole-brain voxel level. In addition, the mean quantitative values of the significant regions were extracted to perform Pearson’s correlation analysis with age in all subjects and with blindness duration in EBA group.Results
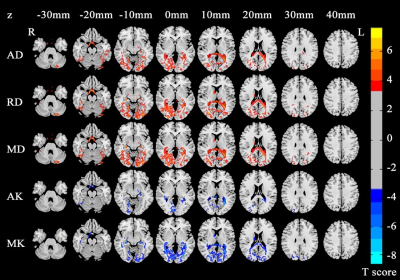
Our results revealed significant increased AD, RD, MD and decreased AK, MK in EBAs compared with NSCs, while no differences were found in FA, RK and KFA maps between the two groups (p < 0.05, FDR corrected, cluster size >100 voxels). The increased tensor metrics were mainly located in bilateral hippocampus, right frontal lobe, left superior occipital gyrus, right caudate, and some left cerebellum regions, while the decreased kurtosis metrics were mainly located in left occipital lobe, right calcarine, and right caudate (Table 1, Figure 1). Pearson’s correlation analysis showed that no significant correlations were found between each of those quantitative DKI values of aforementioned brain regions and age (p > 0.05) or blindness duration (p > 0.05) in EBAs, but the MK of left occipital lobe in NSCs were positively correlated with age (r = 0.561, p = 0.010) (Figure 2).Discussion and conclusion
As conventional diffusion tensor metrics, AD and RD represent the extent of water diffusion occurred parallel and perpendicular to the fiber, respectively. Increased AD and RD reflect degeneration or deterioration of the axonal and myelin integrity, respectively. MD is the mean value of AD and RD describing the diffusion magnitude. Similarly, AK measures the degree of diffusion hindrance or restriction along the principle water diffused direction. MK is the average of the kurtosis along all directions of diffusion gradients. Decreased kurtosis metrics reveal reduced diffusion restriction and tissue complexity.2 In this study, we found increased AD in left hippocampus and superior occipital gyrus, RD in right frontal lobe and caudate, and MD in right hippocampus and caudate in EBA group compared to NSC group. These disrupted regions might associate with their axonal and myelin deficits in populations with aberrant visual function, which are in line with our previous DTI study on EBAs.3In addition, we found decreased AK in right calcarine, left temporal lobe, hippocampus, and corpus callosum, as well as decreased MK in left occipital lobe, right caudate and parahippocampus. These regions were related to the visual information processing, and even constituted an important part of visual pathway. Specifically, previous evidences observed the key role of right parahippocampus in learning and visuospatial configuration of objects,4 and integrated function of caudate on spatial information with motor behavior and some learning processing.5,6
Furthermore, the correlation analyses showed that MK of left occipital lobe were positively correlated with age in NSCs but not in EBAs. Due to the continuous neural development in brain of adolescents, this result may imply the coexistence of neural reorganization and compensatory development process associated with visual deprivation in EBAs. Overall, our results suggested that the diffusion kurtosis metrics are more sensitive to detect the pathology and development related brain regions than diffusion tensor metrics.
Acknowledgements
No acknowledgement found.References
1. Guglielmetti C, Veraart J, Roelant E, et al. Diffusion kurtosis imaging probes cortical alterations and white matter pathology following cuprizone induced demyelination and spontaneous remyelination. NeuroImage. 2016;125:363-77. Epub 2015/11/04.
2. Blockx I, Verhoye M, Van Audekerke J, et al. Identification and characterization of Huntington related pathology: An in vivo DKI imaging study. Neuroimage. 2012, 63(2): 653-62. Epub 2012/06/25.
3. Zhou Z, Xu J, Shi L, et al. Alterations of the Brain Microstructure and Corresponding Functional Connectivity in Early-Blind Adolescents. Neural Plast. 2019 Feb 24;2019:2747460. eCollection 2019.
4. Bohbot. VD, Allen JJ, Dagher A, et al. Role of the parahippocampal cortex in memory for the configuration but not the identity of objects: converging evidence from patients withvselective thermal lesions and fMRI. Frontiers in Human Neuroscience, 2015, 9:431.
5. Gombkötő P, Rokszin A, Berényi A, et al. Neuronal code of spatial visual information in the caudate nucleus. Neuroscience, 2011, 182: 225–231.
6. Carbon M, Ma Y, Barnes A, et al. Caudate nucleus: influence of dopaminergic input on sequence learning and brain activation in Parkinsonism. NeuroImage, 2004, 21(4):1497–1507.
Figures