4443
Mapping diffusion in aging white matter: A comparison of two free-water modeling approaches1Rotman Research Institute, Baycrest Health Sciences, Toronto, ON, Canada, 2Department of Medical Biophysics, University of Toronto, Toronto, ON, Canada, 3Departments of Psychiatry and Radiology, Brigham and Women's Hospital, Harvard Medical School, Boston, MA, United States
Synopsis
To gain more insight into age-effects on white matter diffusivity, advanced diffusion MRI models attempt to separate the effect of cellular diffusion from extracellular isotropic water movement by modeling the latter as free water (FW). Using these models, increased diffusivity with age is typically attributed to an enlargement of the FW compartment with age. The current study compares the sensitivity of FW to age using two common modeling approaches: single-shell fwDTI and multi-shell NODDI.
Introduction
It is well-established that white matter diffusivity increases with normal adult aging.1 To gain more specific insight into this process, advanced models in diffusion MRI attempt to separate the effect of cellular diffusion from extracellular isotropic water movement by modeling the latter as free water (FW). While the cellular compartment(s) in these models provide insight specific to aging tissue microstructure, it is the FW compartment that is typically attributed to increased diffusivity with age, notably as reported using a two-compartment model (fwDTI)2 and NODDI3 (particularly using UK Biobank data4,5) (as well as a recent AxCaliber-based approach.6) The purpose of the current study is to reconcile reported differences in age-sensitivity of FW by comparing age-related differences in FW between single-shell fwDTI and multi-shell NODDI.Methods
Study ParticipantsAll subjects were participants of the UK Biobank. Subjects were excluded from our study if they reported a neurological, psychological or psychiatric disorder, neurological injury, or history of stroke. Subjects were divided into seven age categories [46-50, 51-55, 56-60, 61-65, 66-70, 71-75, 76-80] years and 100 subjects were randomly selected from each of the seven age categories. Data from 52 of the subjects were deemed unusable by the UK Biobank, and 4 additional data were deemed unusable by our own quality control. The final sample thus consisted of 644 subjects aged 46-80 (54% male, 46% female).
Diffusion MRI Data
Information about dMRI data and processing can be found at biobank.ctsu.ox.ac.uk/crystal/crystal/docs/brain_mri.pdf. In brief, dMRI data were acquired on a Siemens Skyra 3T with 5x b=0, 50x b=1000, 50x b=2000 s/mm2 and corrected for distortions via TOPUP.7 NODDI-based FW was modeled using the AMICO toolbox.8 fwDTI-based FW was modeled with in-house MATLAB code using only the b=0 and b=1000s/mm2 data.9
Data Analysis
FSL’s Tract-Based Spatial Statistics10 was used to obtain a WM skeleton. For voxelwise analysis, a relationship of metric with age defined by p<0.05 with correction for multiple comparisons (cluster-wise controlling family-wise error rate with 5000 permutations) and controlling for gender as per FSL’s randomise.11 Age-related difference per year of each FW was calculated as the regression coefficient from a general linear model as implemented in FreeSurfer.
Results
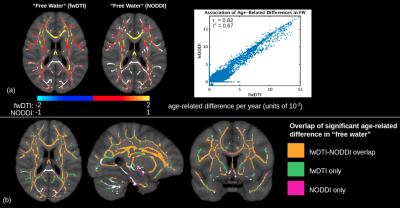
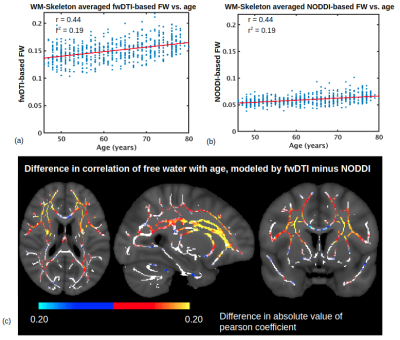
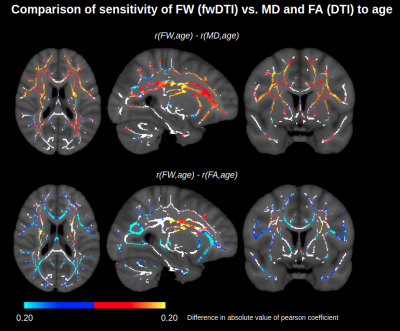
A comparison of FW modeled using fwDTI (based on single-shell data) and NODDI (based on two-shell data) is displayed in Figure 1. The two FW measures are each positively associated with age throughout the WM skeleton, and their age-related differences are highly correlated voxelwise (r=0.82). Figure 2 shows that FW modeled by fwDTI and NODDI both significantly correlate with age when averaged across the WM skeleton. However, the actual values of FW modeled by NODDI (mostly signal fractions between 0.05 and 0.10) are lower than the values computed by fwDTI (mostly between 0.10 and 0.20), and thus the average age-related increase in free water per year is also lower when computed by NODDI (3.9x10-4 per year) than when computed by fwDTI (8.3x10-4 per year). Nonetheless, when normalized by mean, the WM-skeleton averaged age-related increase in FW is 0.65% of mean per year for NODDI and 0.55% of mean per year for fwDTI.On a voxelwise level, FW modeled by fwDTI is more strongly correlated with age than FW modeled by NODDI in 76% of the voxels where both FW exhibit significant age-related differences. Figure 3 shows that fwDTI-based FW is also more strongly correlated with age than mean diffusivity (MD) in 76% of the WM-skeleton voxels manifesting significant associations of both MD and FW with age, but less strongly correlated with age than fractional anisotropy (FA) in 85% of WM-skeleton voxels manifesting significant associations of both FA and FW with age.
Discussion and Conclusion
This study establishes agreement between FW obtained by the two common modeling approaches, single-shell fwDTI and multi-shell NODDI. While previous studies have individually demonstrated that FW is positively associated with age using either approach, this work reconciles these studies by demonstrating that the age-related differences in FW between the two approaches are highly correlated, despite the actual values of each FW differing. Note that the actual values of FW were found to be higher when modeled by fwDTI than NODDI, contrasting findings from a previous preliminary study.12It was also found that FW modeled by fwDTI is often more sensitive to age than when modeled by NODDI, and is also more sensitive to age than MD through much of the WM. Therefore, modeling increased diffusivity with age as FW holds promise as a marker of white matter aging process, which can be derived from either single- or multi-shell diffusion MRI.
Acknowledgements
This research has been conducted using the UK Biobank Resource under Application Number 40922. We thank CIHR, NSERC and NIH for financial support.
References
1. Madden DJ et al (2012) Diffusion tensor imaging of cerebral white matter integrity in cognitive aging. Biochim Biophys Acta 1822(3):386-400.
2. Chad JA et al (2018) Re-examining age-related differences in white matter microstructure with free-water corrected diffusion tensor imaging. Neurobiol Aging 71:161-170.
3. Billiet T et al (2015) Age-related microstructural differences quantified using myelin water imaging and advanced diffusion MRI. Neurobiol Aging 36(6):2107-2121.
4. Cox et al (2016) Ageing and brain white matter structure in 3,513 UK Biobank participants. Nat Commun 7:13629.
5. Miller et al (2016) Multimodal population brain imaging in the UK Biobank prospective epidemiological study. Nat Neurosci 19(11):1523-1536.
6. Fan et al (2019) Age-related alterations in axonal microstructure in the corpus callosum measured by high-gradient diffusion MRI. Neuroimage 191:325-336.
7. Andersson JL et al (2003) How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage 20(2):870-888.
8. Daducci A et al (2015) Accelerated Microstructure Imaging via Convex Optimization (AMICO) from diffusion MRI data. Neuroimage 105:32-44.
9. Pasternak O et al (2009) Free water elimination and mapping from diffusion MRI. Magn Reson Med 62(3):717-730.
10. Smith SM et al (2006) Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 31(4):1487-1505.
11. Winkler AM et al (2014) Permutation inference for the general linear model. Neuroimage 92:381-397.
12. Chad JA et al (2019) Free water mapping in diffusion MRI: How do two common approaches compare? ISMRM 2019:3656.
Figures