4411
Spatiotemporal evolution of ischemic lesions in stroke animal models using free-water elimination and mapping with explicit T2 modelling1INM-4, Forschungszentrum Jülich, Jülich, Germany, 2Institute of Biomedical Engineering and Nanomedicine, National Health Research Institutes, Miaoli, Taiwan, 3Center for Neuropsychiatric Research, National Health Research Institutes, Miaoli, Taiwan, 4Department of Neurology, RWTH Aachen University, Aachen, Germany, 5JARA – BRAIN – Translational Medicine, RWTH Aachen University, Aachen, Germany, 6Institute of Neuroscience and Medicine 11, JARA, Forschungszentrum Jülich, Jülich, Germany, 7Institute of Medical Device and Imaging, National Taiwan University College of Medicine, Taipei, Taiwan
Synopsis
It is known that excess fluid as a result of vasogenic oedema formation following stroke onset obscures the microstructural characterisation of ischemic tissue by diffusion MRI. DTI-based free water elimination and mapping (FWE) has been proposed as a technique to potentially reduce the partial-volume effect. However, FWE estimation is ill-conditioned, leading to inaccurate results. More recently, it has been shown that the addition of a second dimension spanned by transverse relaxation weighting, mitigates the ill-conditioned problem. We aim here to investigate the latter model in a longitudinal study of MCAo stroke animal models.
Introduction
Free-water elimination (FWE) and mapping has been proposed to reduce the bias in DTI metrics induced by the partial-volume effect with free water.1,2 FWE has been shown to be sensitive to the excess fluid in vasogenic oedema in human brain tumours.1,2 Unfortunately, the FWE parameter estimation problem is ill-conditioned.1,3 However, it has been demonstrated that the addition of a second dimension, spanned by the transverse relaxation time weighting, T2 (FWET2), mitigates the ill-conditioned problem.4,5It has been suggested that the pseudo-normalisation of DTI parameters, starting around 24 hours after stroke onset, is mainly due to the formation of vasogenic oedema.6,7 The aim of this study is to investigate the spatiotemporal evolution of ischemic areas in stroke middle cerebral artery occlusion (MCAo) animal models using FWET2 and to evaluate its potential for the characterisation of tissue microstructure in the presence of vasogenic oedema.
Methods
Theory. The model we adopt here assumes that the diffusion MRI signal originates from two compartments with different transverse relaxation rates and diffusivities. In the slow-exchange limit it reads as4 $$S_\mathrm{FWET_2}(T_\mathrm{E},b,\mathbf{n})=S_0[f_\mathrm{w}e^{-T_\mathrm{E}/T_{2,\mathrm{w}}}e^{-bD_\mathrm{w}}+(1-f_\mathrm{w})e^{-T_\mathrm{E}/T_{2,\mathrm{t}}}e^{-b\mathbf{n}^\mathrm{T}\mathbf{D}_\mathrm{t}\mathbf{n}}]\;\;\;(1)$$ where $$$S_0$$$ is the proton density, $$$f_\mathrm{w}$$$, $$$T_{2,\mathrm{w}}$$$ and $$$D_\mathrm{w}$$$ are the fraction, transverse relaxation time and diffusion coefficient of the free-water compartment and $$$T_\mathrm{2,t}$$$ and $$$\mathbf{D}_\mathrm{t}$$$ are the transverse relaxation time diffusion tensor for the tissue compartment. The strength and direction of the diffusion weighting gradient are $$$b$$$ and $$$\mathbf{n}$$$, respectively, and $$$T_\mathrm{E}$$$ is the echo-time. We assume that the vasogenic oedema can be modelled by the isotropic, Gaussian compartment.1,2Animal model. Nine adult, male Sprague–Dawley rats, weighting 350-450 g, were used. All procedures were approved by the Animal Care and Use Committee, National Health Research Institutes, Taiwan. After the pre-stroke scans, rats underwent the MCAo for 90 minutes as described previously.12 Three groups of animals were measured at the following time points: group1{pre,1h,23h,45h,23d} (3 animals); group 2 {pre,11h,33h,23d} (2 animals); group 3 {pre,1d,3d,4d,5d,6d,7d,10d} (4 animals) (h=hours; d=days).
MRI experiments. Experiments were performed on a home-integrated 3T whole-body MRI scanner including an ultra-high-strength gradient coil with a maximum strength of 675 mT/m.8 A custom-designed, single-loop transmit/receive surface coil was utilised. A Stejskal-Tanner, segmented EPI pulse sequence was implemented in-house. Experimental parameters were: TE=50ms and 100ms; b-values(directions) = 0(16), 0.5(24) and 1.0(52) ms/µm2; diffusion gradient separation and duration, Δ=24ms and δ=3ms. Additionally, four more echo-times, TE=70,90,110,130ms (8 repetitions) were acquired. Other parameters were voxel-size=0.26×0.26×13mm3; matrix-size=96×96×20; repetition-time, TR=9s. A turbo spin-echo sequence was used to acquire structural images. Protocol parameters were: TR=4s; TE=68ms; voxel-size=0.13×0.13×1mm3; matrix-size=192×192×20.
Data analysis. Following denoising9 and EPI and eddy-current distortion correction,10 all datasets were analysed using conventional DTI with an explicit, monoexponential T2 attenuation (DTIT2), where the signal is given by: $$$S_\mathrm{DTIT_2}(T_\mathrm{E},b,\mathbf{n})=S_0e^{-T_\mathrm{E}/T_2}e^{-b\mathbf{n}^\mathrm{T}\mathbf{Dn}}$$$. DTIT2 model parameters were estimated using the weighted-linear least-squares estimator. FWET2 model parameters were then estimated using the non-linear least-squares estimator, with the DTIT2 parameters as the initial guess. All estimations were performed using in-house Matlab scripts. Free water parameters were fixed to: $$$T_{2,\mathrm{w}} = 1.25\;\mathrm{s}$$$ and $$$D_\mathrm{w} = 3\;\mathrm{μm^2/ms}$$$.11
Results and discussions
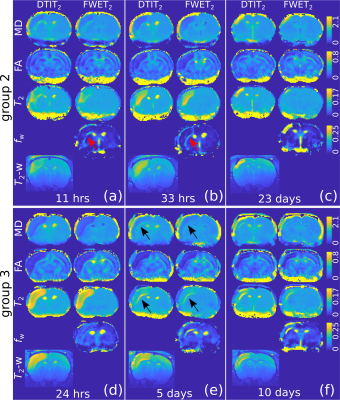
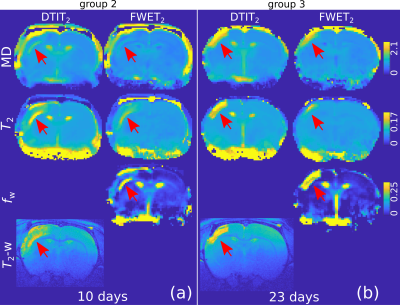
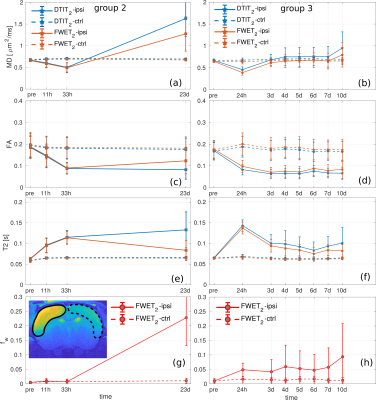
Fig. 1 shows DTIT2 and FWET2 maps for three selected time points for one representative animal from group 2 (a-c) and one representative animal from group 3 (d-f), as an example. A reduction of MD and FA, together with an increase in $$$T_2$$$, is observed up to 33 h after occlusion (Figs. 1a,b,d). Here, the difference between DTIT2 and FWET2 is minor, except for a slight increase in the free water fraction, $$$f_\mathrm{w}$$$, in the white matter (WM) at the ipsi-lateral side (red arrows, Figs.1a,b). At five days (Fig.1e) MD and $$$T_2$$$ tend to re-normalise (black arrows) and $$$f_\mathrm{w}$$$ in WM increases further. At 10 days (Fig.1f) MD and $$$T_2$$$ from DTIT2 tend to increase. However, this is mostly due to the presence of excess fluid, which is reflected by the increase in $$$f_\mathrm{w}$$$ (zoomed maps, Fig.2a) and becomes more heterogeneous. At 23 days (Fig.1c), MD and $$$T_2$$$ from DTIT2 show much higher values, which is reflected by the increase in $$$f_\mathrm{w}$$$ (red arrows, Fig.2b). MD and $$$T_2$$$ from FWET2, which are not contaminated by free water, show much closer values to the contra-lateral side.Fig. 3 shows the group-averaged temporal evolution of MD (a,b), FA (c,d), $$$T_2$$$ (e,f) and $$$f_\mathrm{w}$$$ (g,h) for both DTIT2 (blue) and FWET2 (red), for groups 2 (a,c,e,g) and 3 (b,d,f,h). During the acute phase, MD and FA are reduced and $$$T_2$$$ increases up to 33 hours after occlusion. Here the difference between DTIT2 and FWET2 is not significant. At three days, DTIT2 parameters start showing increasing differences. These differences are partly reflected by the increase in $$$f_\mathrm{w}$$$ (Figs. 3g,h), leading to a reduction in MD and $$$T_2$$$ from FWET2.
Conclusions
We demonstrated the application of FWET2 for the investigation of the spatiotemporal evolution of ischemic lesions in MCAo stroke animal models. We found that, starting at nearly 3 days after stroke onset, the increase in excess fluid in the affected area induces changes in diffusion and relaxation parameters, which are reflected by the free water parameter, $$$f_\mathrm{w}$$$. Thus, FWET2 is potentially helpful to investigate the evolution of vasogenic oedema and tissue-specific features, such as the ischemic penumbra and tissue heterogeneity during the chronic phase.Acknowledgements
We thank Mrs Claire Rick for proof reading the manuscript.References
1. Pasternak O, et al. Free Water Elimination and Mapping from Diffusion MRI. Magn Reson Med 2009;62:717-730.
2. Pierpaoli C, et al. Removing CSF Contamination in Brain DT-MRIs by Using a Two-Compartment Tensor Model. Proc Intl Soc Magn Reson Med. 2004;1215.
3. Hoy AR, et al. Optimization of a free water elimination two-compartment model for diffusion tensor imaging. Neuroimage 2014;103:323-333.
4. Collier Q, et al. Solving the free water elimination estimation problem by incorporating T2 relaxation properties. Proc Intl Soc Magn Reson Med 2017;1783.
5. Farrher E, et al. In vivo DTI-based free-water elimination with T2-weighting. Proc Intl Soc Magn Reson Med. 2018;1629.
6. Hui E, et al. Spatiotemporal dynamics of diffusional kurtosis, mean diffusivity and perfusion changes in experimental stroke. Brain Res. 2012;1451:100-9.
7. Knight RA, et al. Temporal evolution of ischemic damage in rat brain measured by proton nuclear magnetic resonance imaging. Stroke. 1991;22(6):802-8.
8. Cho K-H, et al. Development, integration and use of an ultrahigh-strength gradient system on a human size 3T magnet for small animal MRI. PLoS ONE. 2019;14(6):e0217916.
9. Manjon JV, et al. Adaptive Non-Local Means Denoising of MR Images with Spatially Varying Noise Levels. J Magn Reson Imag. 2010;31(1):192–203.
10. Andersson J, et al. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. NeuroImage. 2003; 20(2):870–888.
11. Collier Q, et al. Diffusion Kurtosis Imaging With Free Water Elimination: A Bayesian Estimation Approach. Magn Reson Med. 2018;80(2):802-813.
12. Wu KJ, et al. Transplantation of Human Placenta-Derived Multipotent Stem Cells Reduces Ischemic Brain Injury in Adult Rats. Cell Transplantation. 2015;24(3):459–470.
Figures