4403
Self-validation for deep learning–based diffusion kurtosis imaging harmonization1Center for Brain Imaging Science and Technology, Department of Biomedical Engineering, Zhejiang University, Hangzhou, China, 2MR Collaboration, Siemens Healthcare, Shanghai, China, 3Department of Imaging Sciences, University of Rochester, Rochester, NY, United States
Synopsis
Deep learning–based harmonization for diffusion imaging data with high efficiency and low cost is gaining popularity. However, the performance of the training-required network depends on the training data, which lack the diversity of the large sets of data in more substantial multicenter projects. We proposed a leave-one-tissue-out training strategy to evaluate the validity and reliability across scanners of a deep learning–based diffusion kurtosis imaging harmonization method. The results confirm that the deep learning–based network can still reconstruct the untrained tissue with validity, although the reliability would be higher when the tissue is trained.
Motivation
Harmonization of diffusion magnetic resonance imaging (dMRI) data in multicenter projects may be able to resolve the data heterogeneity problem across centers caused by different hardware and software1–6. Methods based on deep learning that aims to harmonize the diffusion data with high efficiency and low cost are gaining popularity7–9. However, the performance of the training-required network depends on the training data. Generally, in large multicenter projects, the diversity of testing data is inevitably much greater than the training data. In practice, when the microstructural diffusion tissues from testing subjects are not trained by the deep learning network, the validity and reliability of the outcome remain questionable.In this study, a leave-one-tissue-out (LOTO) training strategy was designed as self-validation for testing the validity and reliability of the network within one untrained tissue. A deep learning–based method that harmonized the diffusion kurtosis imaging (DKI) metrics across scanners was evaluated by several LOTO strategies. Furthermore, another harmonization method of non-deep learning that requires training data like the rotation invariant spherical harmonic (RISH) harmonization5,6,10 was also evaluated and compared with the deep learning–based method.
Methods
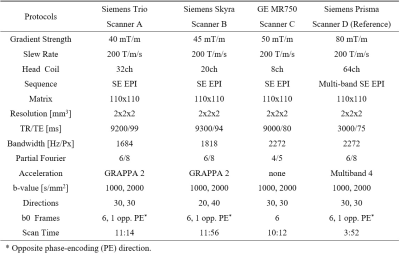
The dMRI data were collected from five healthy traveling volunteers (25.4 ± 1.7 years; two males) who were scanned on four 3T MRI scanners and with different protocols, as listed in Table 1. Scanner D was used as the reference scanner. Data from four subjects were used for training, and one for testing.The harmonization method adapted from a three-dimensional hierarchical convolutional neural network11,12 was applied for joint DKI reconstruction and harmonization in a multicenter study. The neural network for each target scanner was trained by diffusion-weighted images (DWIs) from the target scanner and DKI labels (MK, AK, RK, KFA, and four relevant DTI metrics) from the reference scanner on same subjects. Next, the harmonized DKI metrics were predicted by the network from the DWIs on other subjects from the target scanner.
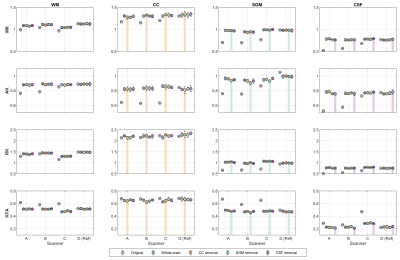
Three LOTO strategies were applied on three tissue types with distinct kurtosis diffusivities from most of the white matter (WM), such as the corpus callosum (CC) of high RK and KFA, subcortical grey matter (SGM) of median kurtosis, and cerebrospinal fluid (CSF) of high diffusivity and low kurtosis, as shown in Fig.1. For each LOTO strategy, the region of one tissue was masked out from both DWIs and DKI labels during the network training, but the whole-brain mask for testing DWIs was unchanged. After the testing, the harmonized DKI metrics from three LOTO strategies were compared with the ones from whole-brain training strategy. Each training was repeated five times for the evaluation.
To make a comparison with the RISH method, the same LOTO strategy on SGM was trained. The DWIs for training and testing were kept consistent with the deep learning–based method, except for the DKI metrices, which were reconstructed after the two single-shell DWIs were separately harmonized. The DKI consistency among the scanners were then evaluated within SGM.
Results and Discussions
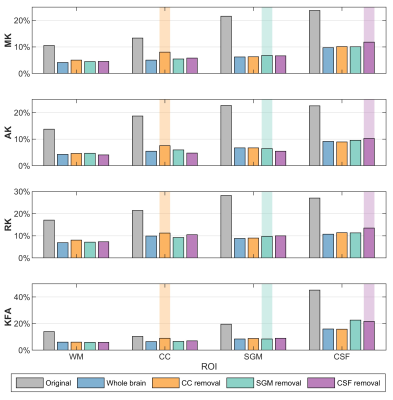
To evaluate the validity and reliability of DKI metrics from different training strategies, the mean values with standard deviations (SDs) within each region-of-interest (ROI) from repeated trainings were obtained (Fig.2). Compared with the original DKI metrics, the harmonized metrics were closer to the values of the reference scanner. For the tissues included in the training stage, such as the WM, the four DKI metrics showed high consistency across all training strategies. Within the ROIs of the untrained tissue, such as the CC in the LOTO training on CC, the relative differences with the whole-brain training were 2.3%, 0.7%, 2.7%, and 2.3% for MK, AK, RK, and KFA, respectively. These differences were similar but at a low level with SGM (0.3%, 2.3%, 1.4%, and 2.8%) and CSF (1.6%, 2.2%, 1.1%, and 4.1%) in their LOTO strategies. Moreover, although the SDs were higher in the tissue which was not trained, the DKI values were apparently different with other tissues.To compare the inter-scanner reliability of harmonized DKI metrics from the LOTO strategies, Fig.3 shows the coefficients of variation (CVs) of original and harmonized DKI across scanners within different ROIs. For the WM, the CVs were substantially reduced after harmonization and were similar across all training strategies. However, in the LOTO strategies, the CV within the untrained tissue was slightly higher than in other training strategies, such as the CC and CSF.
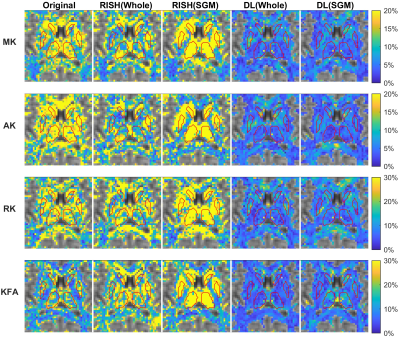
The CV maps are shown in Fig.4 to compare the deep learning–based harmonization with RISH. The RISH method with whole-brain training was capable of improving the coherence of DKI metrics among scanners within the WM and SGM, particularly in the AK. However, in the LOTO strategy, the prediction of the signal within SGM failed with extremely large inter-scanner variance when the SGM was masked out in the training. On the contrary, the deep learning–based method still performed well in both whole brain and LOTO training; also, the CV within the SGM was slightly higher than in the KFA.
Conclusion
The deep learning–based method was able to reconstruct valid and reliable DKI metrics in untrained tissue, where the features could be involved in other trained tissues. The reliability would be higher when the tissues were included in the training.Acknowledgements
We would like to acknowledge the Institute of Neuroscience from Chinese Academy of Sciences, the Third Affiliated Hospital of Qiqihar Medical University, and Zhejiang Hospital for participation in this work.References
1. Pohl KM, Sullivan E V., Rohlfing T, et al. Harmonizing DTI measurements across scanners to examine the development of white matter microstructure in 803 adolescents of the NCANDA study. Neuroimage. 2016;130:194-213.
2. Fortin JP, Parker D, Tunç B, et al. Harmonization of multi-site diffusion tensor imaging data. Neuroimage. 2017;161(July):149-170.
3. Venkatraman VK, Gonzalez CE, Landman B, et al. Region of interest correction factors improve reliability of diffusion imaging measures within and across scanners and field strengths. Neuroimage. 2015;119:406-416.
4. Huynh KM, Chen G, Wu Y, Shen D, Yap P-T. Multi-Site Harmonization of Diffusion MRI Data via Method of Moments. IEEE Trans Med Imaging. 2019;38(7):1599-1609.
5. Mirzaalian H, Ning L, Savadjiev P, et al. Inter-site and inter-scanner diffusion MRI data harmonization. Neuroimage. 2016;135:311-323.
6. Mirzaalian H, Ning L, Savadjiev P, et al. Multi-site harmonization of diffusion MRI data in a registration framework. Brain Imaging Behav. 2018;12(1):284-295.
7. Tax CM, Grussu F, Kaden E, et al. Cross-scanner and cross-protocol diffusion MRI data harmonisation: A benchmark database and evaluation of algorithms. Neuroimage. 2019;195(January):285-299.
8. Ning L, Bonet-Carne E, Grussu F, et al. Muti-shell Diffusion MRI Harmonisation and Enhancement Challenge (MUSHAC): Progress and Results. Computational Diffusion MRI. Cham: Springer International Publishing; 2019:217-224.
9. Koppers S, Bloy L, Berman JI, Tax CMW, Edgar JC, Merhof D. Spherical Harmonic Residual Network for Diffusion Signal Harmonization. Computational Diffusion MRI. Cham: Springer International Publishing; 2019:173-182.
10. Cetin Karayumak S, Bouix S, Ning L, et al. Retrospective harmonization of multi-site diffusion MRI data acquired with different acquisition parameters. Neuroimage. 2019;184(May 2018):180-200.
11. Li Z, Gong T, Lin Z, et al. Fast and Robust Diffusion Kurtosis Parametric Mapping Using a Three-dimensional Convolutional Neural Network. IEEE Access. 2019;7(c):71398-71411.
12. Gong T, He H, Li Z, et al. Efficient Reconstruction of Diffusion Kurtosis Imaging Based on a Hierarchical Convolutional Neural Network. Joint Annual Meeting ISMRM-ESMRMB 2018:1653.
Figures