4393
An analytical segmented approach for extracting TE independent perfusion fraction in intravoxel incoherent motion (IVIM) MRI1Physics, Texas Center for Superconductivity at University of Houston, Houston, TX, United States, 2Diagnostic and Interventional Radiology, Baylor St. Luke's Medical Center, Houston, TX, United States
Synopsis
Determination of perfusion fraction (f) from IVIM-MRI data is challenging. Recent studies have shown that estimation of f is influenced by echo time (TE). Eliminating thisTE dependency on f requires acquiring data sets at multiple TEs. Here, we propose an analytical segmented approach to correct for the TE dependency on f arising from T2 differences between the tissue and fluid compartments (AS-T2). Numerical simulations predict, and phantom experiments confirm that, -compared to commonly used segmented approaches to estimate f, AS-T2 approach permits the determination of perfusion fraction without TE dependence, and with fewer measurements.
Synopsis
Determination of perfusion fraction (f) from IVIM-MRI data is challenging. Recent studies have shown that estimation of f is influenced by echo time (TE). Eliminating thisTE dependency on f requires acquiring data sets at multiple TEs. Here, we propose an analytical segmented approach to correct for the TE dependency on f arising from T2 differences between the tissue and fluid compartments (AS-T2). Numerical simulations predict, and phantom experiments confirm that, compared to commonly used segmented approaches to estimate f, AS-T2 approach permits the determination of perfusion fraction without TE dependence, and with fewer measurements.Introduction
For decades, the two compartment model of tissue diffusion proposed by LeBihan et al1. has held the promise of providing valuable insights into various pathological processes 2-5. However, determination of IVIM model parameters related to perfusion – perfusion fraction (f), and pseudo-diffusion coefficient (Df) has remained a vexing problem 6. Recent studies, have shown that T2 relaxation rate differences between tissue and the fluid compartments can cause significant overestimation of fluid volume fraction (f) as a function of echo time (TE) when using conventional fitting approaches 7,8. We previously proposed an analytical segmented (AS) approach to evaluate f and Df 9. In this work, we extend the AS framework to include T2 correction (AS-T2). The purposes of this works are to: a) describe AS-T2 approach for extracting IVIM model perfusion parameters; (b) verify the feasibility of AS-T2 approach via numerical simulations, and phantom experiments, and (c) compare the performance of AS-T2 approach to widely used segmented (S) and over segmented (OS) approaches for extracting IVIM model parameters.METHODS
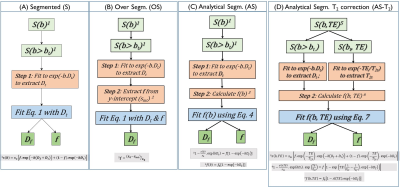
Theory: IVIM based experiments are designed to attenuate MR signal due to diffusivity of tissue and fluid compartments (Dt, and Df), in addition to signal attenuation due to T2 relaxation. Conventional segmented (S) and over segmented (S) approaches as well as analytical segmented (AS) approaches extract IVIM model parameters by implicitly assuming that the T2 relaxation rates of the tissue and fluid compartments are similar, and this affects the estimation of f. The algorithmic steps of each method are pictorially represented in Figure 1.By sampling MR signal over the b, TE space [S(b, TE)], it is possible to estimate f that is independent of TE (Eq. 5, Figure 1). Briefly, in AS-T2 method:
(1) Dt is estimated by fitting the DWI images acquired with b-value high enough to overwhelm signal from the fluid compartment (e.g., b > 200 s/mm2) using equation 1.
(2) T2t is estimated by fitting the DWI images acquired at a high b value e.g. b = 200 s/mm2 as a function of TE into a monoexponential function.
(3) By rearranging Eq. 5, we obtain an expression for f (b, TE) [Equation 7, Figure 1]. By fitting f (b, TE), perfusion related parameters (f and Df) can be extracted.
Numerical Simulations: IVIM MR signal was simulated with the following tissue parameters: f = 0.25; Df/Dt= 0.09/ 0.002 mm2/s; T2f/T2t = 97.3/63.5 ms. IVIM-MRI acquisition parameters were: 10 b values (0,10,25,50,75,100,200,300,500,800 s/mm2); 8 TE ranging from 0 to 150 ms. 5000 signal curves with SNR levels of 20 and 50 were created by adding Gaussian noise.
IVIM acquisition: A non-flowing Gd-doped water (T2 of 63.5 ms) served as static tissue model. A flow pump was used to create flow through a fluid (T2 of 97.3 ms) filled cylinder (diameter: 48 mm) with a fluid velocity of 0.7368 mm/s. Under steady state conditions, DWI were acquired with the same 10 b values used in numerical simulations and at 6 TEs (60,70,80,100,120,150 ms). IVIM MRI signal was constructed by combining the measured MR signal from voxels in the static and fluid compartments in proportion to the presumed perfusion fraction (f).
Data Analysis: Perfusion fraction (f) was estimated using each of the four algorithms (Figure 1) from the MR signal s(b, TE) space spanning b, and TE.
RESULTS
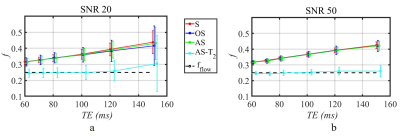
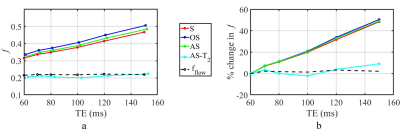
The mean and standard deviation (STD) of estimated f as a function of TE is shown in Figure 2. Conventional fitting approaches and the AS method, consistently overestimate f, by as much as 40% as TE increases from 60 ms to 150 ms.In contrast, AS-T2 method yields an estimate of f value that is independent of TE that matches theory. Experimental results confirm theoretical predictions and numerical simulations (Figure 3).
DISCUSSION AND CONCLUSION
In conclusion, theoretical simulations and phantom experiments suggest that, the proposed AS-T2 approach outperforms conventional segmented and oversegmented analysis methods widely used in clinical settings in the estimation of perfusion fraction. These findings need to be validated in a clinical model.Acknowledgements
No acknowledgement found.References
1. Le Bihan, D., Breton, E., Lallemand, D., Aubin, M. L., Vignaud, J., & Laval-Jeantet, M. (1988). Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology, 168(2), 497-505.
2. Ma W, Zhang G, Ren J, Pan Q, Wen D, Zhong J, Zhang Z, Huan Y. Quantitative parameters of intravoxel incoherent motion diffusion weighted imaging (IVIM-DWI): potential application in predicting pathological grades of pancreatic ductal adenocarcinoma. Quant Imaging Med Surg. 2018 Apr;8(3):301-310. doi: 10.21037/qims.2018.04.08. PMID: 29774183; PMCID: PMC5941211.
3. Federau, C., Sumer, S., Becce, F., Maeder, P., O’Brien, K., Meuli, R., & Wintermark, M. (2014). Intravoxel incoherent motion perfusion imaging in acute stroke: initial clinical experience. Neuroradiology, 56(8), 629-635.
4. Cho, G. Y., Moy, L., Zhang, J. L., Baete, S., Lattanzi, R., Moccaldi, M., ... & Sigmund, E. E. (2015). Comparison of fitting methods and b‐value sampling strategies for intravoxel incoherent motion in breast cancer. Magnetic resonance in medicine, 74(4), 1077-1085.
5. Togao, O., Hiwatashi, A., Yamashita, K., Kikuchi, K., Mizoguchi, M., Yoshimoto, K., ... & Honda, H. (2015). Differentiation of high-grade and low-grade diffuse gliomas by intravoxel incoherent motion MR imaging. Neuro-oncology, 18(1), 132-141.
6. Andreou, A., Koh, D. M., Collins, D. J., Blackledge, M., Wallace, T., Leach, M. O., & Orton, M. R. (2013). Measurement reproducibility of perfusion fraction and pseudodiffusion coefficient derived by intravoxel incoherent motion diffusion-weighted MR imaging in normal liver and metastases. European radiology, 23(2), 428-434.
7. Jerome, N. P., d’Arcy, J. A., Feiweier, T., Koh, D. M., Leach, M. O., Collins, D. J., & Orton, M. R. (2016). Extended T2-IVIM model for correction of TE dependence of pseudo-diffusion volume fraction in clinical diffusion-weighted magnetic resonance imaging. Physics in medicine and biology, 61(24), N667.
8. Qu, F., Hor, P. H., Fischer, J., & Muthupillai, R. (2018). Tissue characterization of uterine fibroids with an intravoxel incoherent motion model: The need for T2 correction. Journal of Magnetic Resonance Imaging, 48(4), 994-1001.
9. Buko, E., Zhang, J., Ajala, A., Hor, P.H, & Muthupillai, R. (2018). An Analytical Segmented (AS) Approach for Extracting IntraVoxel Incoherent Motion (IVIM) Model Parameters. Joint annual meeting ISMRM-ESMRMB 2018 p. 5361
Figures