4386
Synthetic-DWI with T2-based Water Suppression1Department of Radiological Science, Shizuoka College of Medicare Science, Hamamatsu-Shi, Japan
Synopsis
We proposed a new Diffusion Weighted Imaging (DWI) with T2-based water suppression technique (Wsup-DWI) and additionally combined with synthetic-MRI technique. Our water suppression was achieved by subtracting heavy T2-weighted (long-TE) image from the standard DWI images after correcting signal intensities due to b-value. We demonstrated that hyperintense artifacts due to CSF partial volume effects (PVE) were dramatically suppressed even at lower b-values (b); and quantitative maps of ADC and FA became close to the tissue values due to CSF reduction. Furthermore, fiber tracts became longer for Wsup-DWI data than for standard-DWI data in a tractography with the same condition.
Introduction
CSF suppression is very important for DWI to obtain quantitative maps such as ADC, FA, and tractography in brain tissue. This is because those are affected by partial volume effect (PVE) if CSF is mixed in a voxel. To solve those problems, various CSF suppression techniques for DWI have been proposed [1]. In those, FLAIR-DWI [2, 3] has problems of SNR reduction, extended imaging time, and inability to obtain water information. Analytical approaches to separate free-water and tissue components (FWS) [4, 5] proposed; however, they requires many data points of different b-values and the diffusion gradient directions, and are sometimes the results are instable. The 3rd approaches to use greater b-values of reducing water signals (e.g. b>400 s/mm2) (NZE) [6, 7] have problems of tissue SNR reduction and insufficient water suppression. The purpose of this study was to propose and assess a new water suppression technique for DWI to solve those problems existing in conventional methods, and the combination with our water suppressed synthetic MRI technique [9].Methods
TheorySchematic of two-compartment model for DWI signal are shown in Fig.1. Here we consider the problems of simply obtaining water-suppressed DWI (Wsup-DWI) images and parameter maps of tissue. A T2-based water suppression technique [8, 9] was applied to reduce the CSF-PVE in DWI.
A Total flow of water suppression for DWI is as follows. Standard Spin-echo (SE)-DWI sequence is used in imaging. First, heavy-T2W (long-TE) images, Sw are acquired in addition to images with b-value (b [s/mm2]) of zero (b0), and standard DWI images with bn>0, S. Water images with acquired b-values are obtained by using water ADC (ADCw), where ADCw is measured at stationary pure water (CSF) portions or assumed to be literature value of ~3.0x10-3 [mm2/s]. Second, Wsup-DWI images, Swsup (~St ) are obtained by weighted subtraction of corrected long-TE images from the corresponding standard acquired DWI images, S with the same b-value as:
Swsup (TE,bn )=S(TE,bn ) - α(TE)∙Mask∙Sw(TElong,b0)∙exp[-(bn-b0)∙ADCw],
where α(TE) is a scaling factor for T2 decay correction of water signal, and Mask is a spatial mask to keep SNR on tissue portions after subtraction . Finally, quantitative maps of ADC and FA or tractography are obtained using Wsup-DWI images for whole range of b-values including b=0.
MRI Experiments
In MR experiments, the data (2 or 3 TEs with b0~0 and 1 TE with bn>0 ) in Fig. 1 were acquired for our proposed Wsup-Syn.MRI. A healthy volunteer study was performed on a MRI (‘Galan 3T ZGO’, Canon Medical Systems corp., Japan) with a 32-channel head coil after obtaining informed consent. A spin-echo (SE) DWI sequence was used and the common acquisition parameters were: parallel imaging of speed-up factor 3, the number of slices was selected at maximum, NAQ=1, acquisition matrix=192 x 256 (PE x RO), display matrix=512 x 512 after sinc interpolation.
Following 2 kinds of data were used:
Case-1 (3-axis DWI): 5mm x 16slices, TR=8000ms, TE1=25ms, TE2=80ms, TElong=300ms, TI=1000ms, b[s/mm2]= 0, 250, 500, 1000, 2000. Images of isotropic DWI and ADC were obtained for various b-value combinations in addition to Wsup-synthetic images of b=0.
Case-2 (6-axis DTI): 3mm x 50slices, TR=10000ms, TElong=500ms, TE2=48ms, b[s/mm2]= 0, 1000. Images of isotropic DWI, ADC, and FA, and tractography were obtained. ‘DSI studio’ (http://dsi-studio.labsolver.org) was used for DTI data analysis.
Results
Resulting DWI images for case-1 (Fig. 2) and those ADC maps including contrast weighted images and quantitative parameter maps (Fig. 3) for Standard (w/o Wsup) and for our proposed Water suppression (Wsup). the CSF signals for Wsup were almost perfectly suppressed in whole range of b-value including b=0, especially in PVE portions such as brain cortex and cerebellum (Fig. 2).Characteristics of ln[S(b)] vs. b, in PVE portions for Standard were not linear due to CSF-PVE, in contrast, those for Wsup have higher linearity and the gradients corresponding to ADC values became close to the tissue values (Fig. 4).
Regarding the DTI results for case-2 at CSF-PVE portions (Fig. 5), ADC values became lower and FA values became close to 1 in Wsup data; and in addition, tracts in Wsup data became longer as a result of greater FA values.
Discussion
Regarding the tissue SNR after water suppression, it can be estimated that our Wsup-DWI is better than the FLAIR-DWI because it has already verified that the Wsup b0 image has higher SNR [8, 9]; and it was shown in Fig. 3 that our Wsup-DWI is better than the NZE-DWI. Furthermore, the b~500 used in NZE-DWI was not sufficient, since b>1000 was required to perfectly reduce CSF signals in our results.The biggest advantage in our method is that whole range of b-value (b>0) can be applied in calculation of DWI/DTI parameters. Regarding the FWS method, although it has not compared yet, it is clear that our Wsup method has an advantage in imaging time and analysis simplicity.
Conclusion
Although further optimization of pulse-sequence and processing techniques, and clinical assessments especially for long T2 lesion, our proposed synthetic DWI technique with T2-based water suppression will solve the problem of CSF-PVE artifacts in current DWI more easily and higher quality than the other water suppression methods already proposed, and it is expected to become further clinically useful.Acknowledgements
We express our sincere thanks to Yuki Takai and Ryo Shiroishi in Canon Medical Systems Corporation for supporting data acquisition and analysis in this study.References
[1] Salminen LE, et al. Reducing CSF Partial Volume Effects to Enhance Diffusion Tensor Imaging Metrics of Brain Microstructure. Technol Innov. 2016;18:5-20.
[2] Latour LL, et al. Cerebral spinal fluid contamination of the measurement of the apparent diffusion coefficient of water in acute stroke. Magn Reson Med. 2002;48(3):478–486.
[3] Papadakis NG, et al. Study of the effect of CSF suppression on white matter diffusion anisotropy mapping of healthy human brain. Magn Reson Med. 2002;48(2):394–398.
[4] Pasternak O, et al. Free water elimination and mapping from diffusion MRI. Magn Reson Med. 2009;62(3):717–730.
[5] Pasternak O, et al. Estimation of extracellular volume from regularized multi-shell diffusion MRI. Med Image Comput Comput Assist Interv. 2012;15(2):305–312.
[6] Baron CA, et al. Acquisition strategy to reduce cerebrospinal fluid partial volume effects for improved DTI tractography. Magn. Res. Med. 2015;73(3):1075–1084.
[7] Salminen LE, et al. Phillips S, Paul RH. Regional age differences in gray matter diffusivity among healthy older adults. Brain Imag Behav. 2016;10(1):203–211.
[8] Kimura T et al. Synthetic MRI with water suppression technique to reduce CSF partial-volume dependent artifacts in brain. ISMRM, 27th 2019: #4867.
[9] Essig M et al. Assessment of cerebral gliomas by a new dark fluid sequence, high intensity REduction (HIRE): a preliminary study. JMRI11:506-517(2000)
Figures
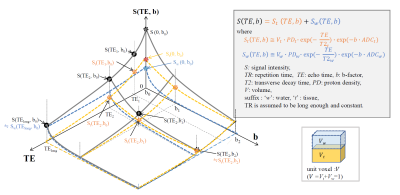
Fig. 1 SE-DWI signal space, S(TE,b) based on a two-compartment model in a unit voxel contains tissue and water.
The minimum number of data to obtain T2t and ADCt is 4 points, S(TE1,b0), S(TE2,b0) ,and S(TElong,b0) and additionally S(TE2,b1) where TElong is selected as a water signal dominant; and 3 points except for S(TE1,b0) to obtain only ADCt. Several gradient directions for b1 are required to obtain diffusion directions; e,g. 3 for isotropic DWI and ADC, or 6 for DTI.
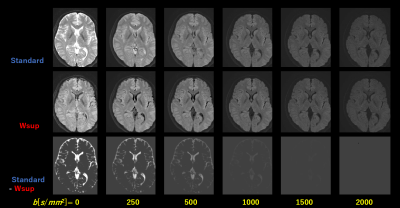
Fig. 2 DWI images in Case-1 as a parameter of b-value for Standard (w/o Wsup) and for proposed Water suppression (Wsup), and those subtracted images, where display windows are all the same.
Note that CSF signals in Wsup were well suppressed even at small b-values (< 500), though CSF signals for Standard were almost decreased with increasing b-value (>1000 ).
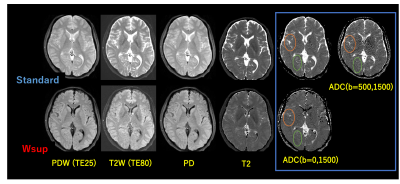
Fig. 3 Contrast-weighted images and parameter maps for Standard vs. Wsup in Case-1.
CSF-PVE artifacts shown in Standard were well reduced in Wsup, especially at temporal cortex and cerebellum. Although the CSF-PVE artifacts were reduced in Standard ADC with b=(500,1500) which corresponds to NZE-DWI method, but still remained compared to our proposed Wsup with b=(0,1500) (circles), and furthermore, tissue SNR for the Wsup with b=(0,1500) were better than the Standard of b=(500,1500).
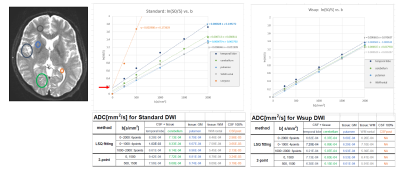
Fig. 4 DWI ROI analysis results in Case-1 for Standard and Wsup: ln[S(b)] vs. b (top) and ADC values with several method (bottom).
Regarding the results of linear fitting by ln[S(b)]=A*b+B, The B for standard was not zero (red allow) reflecting the CSF-PVE, in contrast, the B for Wsup was almost zero. The A corresponding to ADC value at the portions existing CSF-PVE (cerebram, temporal lobe) for Wsup were close to pure tissue (putamen) values in any range of b-value combinations. The ADC value of pure CSF portion in the Standard was close to literature value.
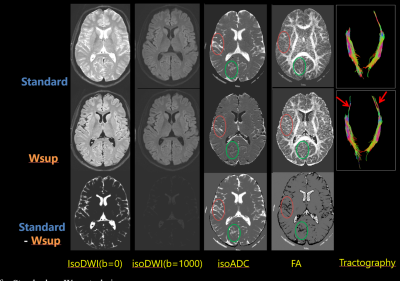
Fig. 5 DTI results in Case-2 for Standard vs. Wsup technique.
Note that, CSF-PVE artifacts in Wsup were reduced compared to the Standard especially at temporal cortex or cerebellum; i.e., the ADC was reduced and the FA was increased, and the fiber tract length became longer (arrows) in Wsup data. Here the DWI images and the subtractions were shown with the same window.