4383
Quantifying whole-brain microstructural changes in rat model of focal ischemia on unilateral motor cortex using diffusion MRI at 14T1Interdisciplinary Institute of Neuroscience and Technology (ZIINT), College of Biomedical Engineering and Instrument Science, Zhejiang University, HangZhou, China, 2Qiushi Academy for Advanced Studies (QAAS), Key Laboratory of Biomedical Engineering of Ministry of Education, College of Biomedical Engineering and Instrument Science, Zhejiang University, HangZhou, China, 3School of Medicine, Zhejiang Univerisity, HangZhou, China
Synopsis
Focal cortical ischemia animal model has been widely used to study neuroanatomical reorganization of cortex with intracortical microstimulation. However, the whole-brain structural changes following focal ischemia on motor cortex is unknown. In this study, high-resolution DTI is performed on focal unilateral motor cortex ischemia rat model at 14T. Voxel-wise based analysis (VBA) and VBA-guided connectivity analysis are performed to explore potential whole-brain microstructure and global structural connectivity changes. Our results show that large-scale white matter microstructural changes mainly distribute in corpus callosum, external capsule, cerebral peduncle. Significant reorganization of sensorimotor network associated with these regions were also found after ischemia.
Introduction
Motor impairments represent the most common form of disability resulting from stroke. Recovery of motor function after stroke is typically incomplete and challenging. Several new motor rehabilitation therapy strategies, e.g., repetitive Transcranial Magnetic Stimulation, have been proposed and showed promising potentials in helping the recovery of motor function in stroke patients1. However, the plasticity mechanism associated with motor function recovery is still controversial2, which is becoming an insuperable obstacle for the improvements of motor rehabilitation therapy efficacy, for example, the debating stimulation targets3.By using electrophysiology and other invasive methods, numerous studies on focal cortical ischemia animal model show that reorganization of cortical motor representations of the adjunct, intact tissue and diffuse connectivity is essential for functional recovery4, 5. However, little is known whether such a focal cortical lesion could cause whole-brain structural changes. In this study, high-resolution DTI is performed on focal motor cortex ischemia rat model at 14T. Voxel-wise based analysis (VBA) and VBA-guided connectivity analysis are performed to explore the potential whole-brain microstructural and global connectivity changes induced by focal motor cortex ischemia.
Methods
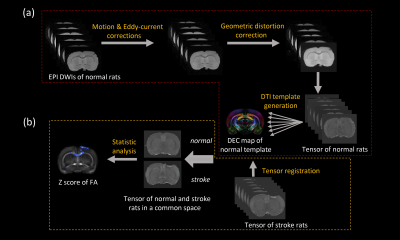
The photothrombotic ischemia (PTI) model was used in our study to induce focal motor cortex ischemia. Twelve adult male Sprague Dawley rats weighted from 230g to 280g were used. Six rats were served as control group. Another six rats were subjected to focal ischemia group and occlusion was induced by focal illumination with 532nm laser for 15min on a 3.5mm diameter cranial window on motor cortex (CFA) after injecting Rose Red dye on caudal veins. Ex vivo PFA-fixed brain were obtained from the ischemia group 4-5 weeks later after ischemia onset. All scanning were performed at 14T vertical Bruker Micro imaging system with a 20 mm RF coil. DTI was performed using three-dimensional echo planar imaging sequence with eight segments, TE/TR = 31/800ms, b = 3800s/mm2, 32 gradient directions, δ/Δ = 3ms/18ms, 0.16×0.16×0.16mm3 voxel size. T2-weighted images were obtained using a three-dimensional multi slice multi echo sequence (MSME) with same spatial dimensions as DWI images, TE/TR = 4/1000ms, 32 echoes.All DWIs pre-processing, DTI template generation, and image registration were preformed within TORTOISE6. Firstly, a DTI population template was built by diffeomorphic tensor-based registration of control group. Secondly, the ischemia group were registered to the template with a tensor-based registration algorithm7. Then, VBA analysis with permutation test was performed to compare FA differences across whole brain. Finally, fiber tractography was performed within MRtrix38.
Results and Discussion
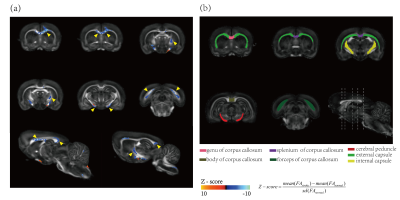
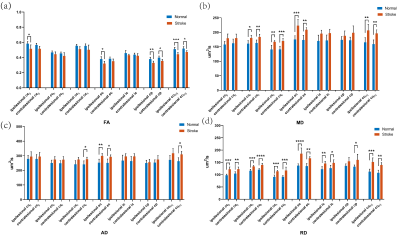
Figure 2 shows VBA results overlaid on the template FA maps. Voxels with significantly changed FA are mainly distributed in seven brain regions, including four sub-regions of corpus callosum (the genu (ccg), the body (ccb), the splenium (ccs), the forceps (ccfmj)), external capsule (ec), internal capsule (ic) and cerebral peduncle (cp), as illustrated with yellow arrows in Fig. 2(a). To further confirm these changes, region-based analysis was also performed on the intact anatomical regions (Fig. 2(b)) drawn manually according to Paxinos and Watson atlas. All the selected regions of the ischemia group show reductions in FA, increases in MD, AD, and RD (Fig. 3), though some changes are not significant with two-way ANOVA analysis, which might due to be the relatively large size of these ROI and the portion of the changed voxels are relatively small. The increases of both AD and RD indicate the loss of fibers in these detected voxels.The altered microstructure properties in the cc indicates the interhemispheric connectivity might be reduced. Our fiber tractography results confirm this: while the overall sensorimotor (SM) network streamline numbers do not show significant differences between the ischemia group and the control group, the interhemispheric SM connectivity is reduced in the stroke group, in which the interhemispheric SM streamlines through cc are significantly reduced by around 29% in ischemia group (Table 1(a). cc-bridged SM network). The reduction of interhemispheric connectivity agrees with previous studies9, 10.
Interestingly, the total intrahemispheric SM network streamline numbers in either hemisphere show a trend of increase (yet not significant). To further understand the origins of these increased intrahemispheric connections, the SM network streamlines passing ec were studied. The SM network streamlines passing ipsilesional ec region of the ischemia group doesn’t show significant changes in total numbers (Table 1(a)), but obvious redistribution of these streamlines’ connections is observed: around 20% streamlines switched from interhemispheric connections to intrahemispheric connections (Table 1(c)). Similar redistribution of streamlines’ connection happens in contralesional ec. We speculated that the increased intrahemispheric connectivity may be related to proliferation of new circuits following Hebbian-type refinement after stroke. Whether the increased intrahemispheric connectivity is beneficial to the motor functional recovery is an interesting future direction to study.
Conclusion
By performing VBA analysis and VBA-guided connectivity analysis, we found focal motor cortex ischemia lesion could induce large-scale white matter microstructural changes in both hemispheres, mainly distributed in cc, ec, and CST-related regions. Significant reorganization of sensorimotor network associated with these regions were found, including reduction of interhemispheric connectivity, and increase of intrahemispheric connectivity in both hemispheres, though the contralesional changes seems weaker. This study could provide useful information for the understanding of the plasticity mechanism of functional recovery and target selection for stimulation-based therapy.Acknowledgements
Images acquistion was supported by the 14T Micro imaging system in Department of Chemistry, Zhejiang University. We greatly thank to Professor Xueqian Kong to provide adequate scanning-hour for us.References
1. Lefaucheur JP. Stroke recovery can be enhanced by using repetitive transcranial magnetic stimulation (rTMS). Neurophysiol Clin 2006; 36: 105-115.
2. Murphy TH and Corbett D. Plasticity during stroke recovery: from synapse to behaviour. Nat Rev Neurosci 2009; 10: 861-872.
3. Plow EB, Cunningham DA, Varnerin N, et al. Rethinking stimulation of the brain in stroke rehabilitation: why higher motor areas might be better alternatives for patients with greater impairments. Neuroscientist 2015; 21: 225-240.
4. Dancause N, Barbay S, Frost SB, et al. Extensive cortical rewiring after brain injury. J Neurosci 2005; 25: 10167-10179.
5. Nudo RJ and Milliken GW. Reorganization of movement representations in primary motor cortex following focal ischemic infarcts in adult squirrel monkeys. J Neurophysiol 1996; 75: 2144-2149.
6. Pierpaoli C, Walker L, Irfanoglu M, et al. TORTOISE: an integrated software package for processing of diffusion MRI data. ISMRM 18th Annual Meeting 2010.
7. Irfanoglu MO, Nayak A, Jenkins J, et al. DR-TAMAS: Diffeomorphic Registration for Tensor Accurate Alignment of Anatomical Structures. Neuroimage 2016; 132: 439-454.
8. Tournier JD, Smith R, Raffelt D, et al. MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. Neuroimage 2019; 202: 116137.
9. van Meer MP, van der Marel K, Wang K, et al. Recovery of sensorimotor function after experimental stroke correlates with restoration of resting-state interhemispheric functional connectivity. J Neurosci 2010; 30: 3964-3972.
10. Wang LE, Tittgemeyer M, Imperati D, et al. Degeneration of corpus callosum and recovery of motor function after stroke: a multimodal magnetic resonance imaging study. Hum Brain Mapp 2012; 33: 2941-2956.
Figures