4373
A Comparison of Image-based and Field-monitoring-based Correction for Eddy Current and Bo Field Distortions in Diffusion Imaging Data1Laboratory for Social and Neural Systems Research (SNS Lab), University of Zurich, Zurich, Switzerland, 2Institute of Biomedical Engineering, ETH, Zurich, Switzerland
Synopsis
Diffusion MRI commonly proceeds with single-shot echo planar imaging (EPI) readout, which renders this modality sensitive to Eddy current and susceptibility-induced distortions. Both image-based and k-space-based correction methods have been suggested previously. The aim of this project was to use a standard dataset and compare output images of two image-based correction methods (topup/Eddy and Tortoise) and the extended signal model method. The latter relies on separate magnetic field monitoring data and a Bo field map. Qualitatively the output of three pipelines were similar. Future work will make the diffusion acquisition more challenging for a more complete systematic evaluation.
Introduction
Diffusion MRI (dMRI) data are commonly acquired with single-shot echo planar imaging (EPI) readout1. While this avoids image artifacts that would result from phase differences between shots, due to its low bandwidth in the phase encoding direction, EPI is sensitive to deviations of the k-space readout trajectory2. In particular, Eddy currents (induced by the diffusion-encoding gradients) last through the 30-50ms readout, and inhomogeneities of the static Bo will distort the local magnetic field gradients from their expected linear modulation. Both image-based3–6 and k-space-based correction methods have been proposed. One variant of the latter is magnetic field monitoring7,8, which relies on field probes9 that concurrently measure the spatio-temporal magnetic field dynamics and use these additional data together with a static Bo map from a multi-echo gradient-echo (GRE) acquisition in an offline reconstruction pipeline10,11. Although the latter method is expected to perform better given that it is not model-based and it has additional data to rely on for the correction, it is time consuming and requires additional equipment set up.Therefore, the aim of this project was to compare the output of Eddy (as implemented in FSL, FMRIB, Oxford, England) and Tortoise with that of an informed reconstruction with an extended signal model.
Methods
Data Acquisition: A healthy adult volunteer was scanned in accordance with local ethics guidelines using a 3T Philips Achieva scanner and 8ch receive-only head coil integrated with 16 NMR field probes12. Two high angular resolution diffusion imaging (HARDI) datasets were acquired with opposite phase-encoding directions at 1.7 mm isotropic resolution. Each HARDI dataset included two b0 images and 61 diffusion-weighted images (DWIs; vectors on sphere; b=2000s/mm2) with TR=10sec, TE=104ms, Stejskal-Tanner diffusion encoding, SENSE factor=2, field-of-view=220x220x104mm3. During each readout period of the HARDI sequence, the magnetic field dynamics up to 3rd order in space were monitored10. In addition, GRE images at 5 echo times (TE1/ΔTE=4.6/1.15ms) were collected for the estimation of static Bo map and coil sensitivity maps.Image reconstruction and processing:
1. Using the concurrently monitored field dynamics, static Bo map, and coil sensitivity maps, each HARDI dataset was reconstructed offline with an expanded signal model13,14. The Bo map corrects susceptibility distortions.
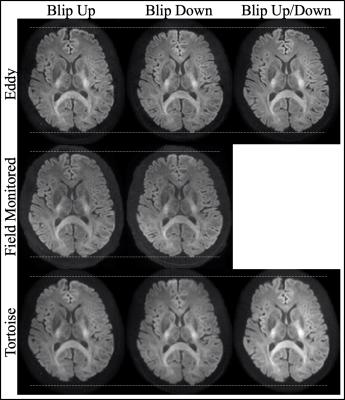
2. Topup and Eddy (FSL version 5.0.11)3 were run three times on two vendor-reconstructed HARDI datasets; once on blip-up HARDI dataset, once on blip-down HARDI dataset, and once combining the two HARDI datasets.
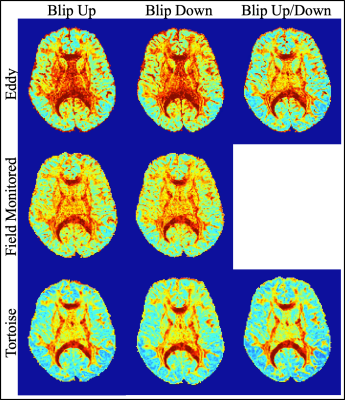
3. Tortoise5,6 were run as topup and Eddy.Data Analysis: The mean and coefficient of variation (CoV) across 61 DWIs were calculated for each distortion-corrected HARDI dataset. The diffusion tensor was estimated on each HARDI dataset using the ordinary linear least squares approach (dtifit from FSL).
Results
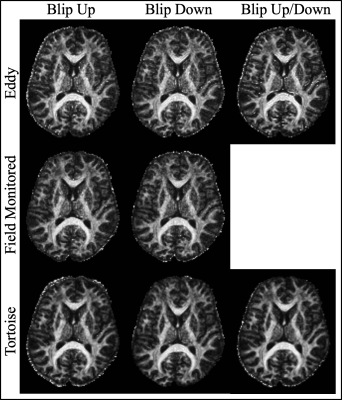
The well-defined boundary between the gray and white matter in the mean of the 61 DWIs indicates that the individual DWIs were each appropriately corrected for Eddy current distortions (Fig_1). Apart from a slightly smoother appearance of the Tortoise output the images looked qualitatively similar. The size of the brain in the phase encoding (i.e. anterior-posterior) direction varied the least with the field monitored reconstruction although that includes the Bo field map. The CoV across the 61 DWIs, particularly at the edges of the brain, is a complimentary test for the congruence of the individual DWIs15 (Fig_2). When the blip up/down data were used separately, Eddy had a slightly higher CoV at the edges of the brain (mostly frontal) which was much reduced when both data sets were combined. It is remarkable that when Eddy had access to only the blip up or down data the CoV was increased in the centre of the brain while both the field monitored reconstruction and Tortoise produced similar CoV for the blip up/down data. The three pipelines also produced similarly high-quality fractional anisotropy images.Discussion
We purposely started this investigation with a relatively easy acquisition protocol (i.e. low res and low b-value) and the image-based and k-space-based correction methods produced images with similar quality. The comparison is inherently unfair, given that field monitoring yields extra data for the correction, albeit most of it simultaneously. Although when Eddy was fed with only the blip up or the blip down data the CoV was increased in the center of the brain (i.e. white matter), this discrepancy was eliminated when both blip up/down data were used.There are pros and cons to both methods. Eddy and Tortoise simultaneously correct for subject motion. Although, the magnetic field probes can be used for motion correction16, the current implementation of the informed reconstruction proceeded without it (we relied on a highly cooperative volunteer for this comparison). On the other hand, concurrent measurement of the magnetic field during the EPI readout with magnetic field probes captures many different sources of the spatio-temporal field deviations and does not rely on modeling these sources. For example, it captures field deviations from gradient heating, vibrations, physiological sources, such as breathing.
Future work will make the acquisitions systematically and progressively more challenging. Increasing the resolution requires longer EPI readout times and/or higher parallel imaging acceleration and will result in lower SNR. Increasing the b-value will not only reduce SNR further but the larger diffusion-encoding gradients also will cause variable Eddy current times and magnitudes.
Acknowledgements
This work was supported by Swiss National Science Foundation (grant nrs: 316030_164076 & 31003A_166118). The authors gratefully acknowledge Dr. Jesper Andersson from FMRIB in Oxford, England as well as Prof. Carlo Pierpaoli and Dr. Mustafa Okan Irfanoglu from the NIH in Bethesda, USA, for assistance with their respective image processing methods and fruitful discussions on the interpretation of the results.References
1. Turner, R.et al.Echo-planar imaging of intravoxel incoherent motion. Radiology177, 407–414 (1990).
2. Fischer, H., Ladebeck, R., Schmitt, F., Stehling, M. K. & Turner, R. Echo-Planar Imaging Image Artifacts. in Echo-Planar Imaging: Theory, Technique and Application179–200 (Springer, 1998).
3. Andersson, J. L. R. & Sotiropoulos, S. N. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage125, 1063–1078 (2016).
4. Andersson, J. L., Skare, S. & Ashburner, J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage20, 870–888 (2003).
5. Pierpaoli, C. M. et al.TORTOISE: an integrated software package for processing of diffusion MRI data. in Proceedings of the 18th Annual meeting of the International Society for Magnetic Resonance in Medicine, Stockholm, Sweden1597 (2010).
6. Irfanoglu, M. O., Nayak, A., Jenkins, J. & Pierpaoli, C. M. TORTOISEv3: Improvements and New Features of the NIH Diffusion MRI Processing Pipeline. in Proceedings of the 275h Annual Meeting of the International Society for Magnetic Resonance in Medicine, Honolulu, USA3540 (2017).
7. Barmet, C., De Zanche, N. & Pruessmann, K. P. Spatiotemporal magnetic field monitoring for MR. Magn Reson Med60, 187–197 (2008).
8. Dietrich, B. E. et al.A field camera for MR sequence monitoring and system analysis. Magn Reson Med(2015). doi:10.1002/mrm.25770
9. De Zanche, N., Barmet, C., Nordmeyer-Massner, J. A. & Pruessmann, K. P. NMR Probes for measuring magnetic fields and field dynamics in MR systems. Magn. Reson. Med.60, 176–186 (2008).
10. Wilm, B. J.et al.Diffusion MRI with concurrent magnetic field monitoring. Magn Reson Med74, 925–933 (2015).
11. Wilm, B. J.et al.Single-shot spiral imaging enabled by an expanded encoding model: Demonstration in diffusion MRI. Magn Reson Med77, 83–91 (2017).
12. Kennedy, M., Lee, Y. & Nagy, Z. An industrial design solution for integrating NMR magnetic field sensors into an MRI scanner. Magn Reson Med80, 833–839 (2018).
13. Wilm, B. J., Barmet, C., Pavan, M. & Pruessmann, K. P. Higher order reconstruction for MRI in the presence of spatiotemporal field perturbations. Magn Reson Med65, 1690–1701 (2011).
14. Wilm, B. J., Barmet, C. & Pruessmann, K. P. Fast higher-order MR image reconstruction using singular-vector separation. IEEE Trans Med Imaging31, 1396–1403 (2012).
15. Tournier, J.-D. D., Mori, S. & Leemans, A. Diffusion tensor imaging and beyond. Magn Reson Med65, 1532–1556 (2011).16. Haeberlin, M. et al.Real-time motion correction using gradient tones and head-mounted NMR field probes. Magn Reson Med74, 647–660 (2014).
Figures