4367
Magnitude-regularized Phase Estimation (MAPE) with U-Net Support for Self-navigated Multi-shot Echo-planar DWI in the Brain1Institute for Signal Processing, University of Luebeck, Luebeck, Germany, 2Philips Research Europe, Hamburg, Germany, 3Department of Radiology, LUMC, Leiden, Netherlands
Synopsis
We propose a self-navigated iterative reconstruction algorithm for multi-shot DWI which effectively performs the shot phase updates with a fixed joint image prior. This framework further nicely incorporates deep learning generated image priors into the shot phase estimation while keeping the joint image production isolated. A U-Net is trained on extra-navigated data to mitigate phase cancellation artifacts. The algorithm with and without U-Net support is compared to self- and extra-navigated reference algorithms. The U-Net approach effectively mitigates phase-related signal cancellation artifacts. The improved multi-shot image prior regularizes the shot phase estimation enabling highly segmented self-navigated diffusion echo-planar imaging.
Introduction
Multi-shot techniques are widely studied to overcome the resolution limits of diffusion-weighted imaging (DWI)1, but these approaches generally suffer from strong shot-specific phase variations2. Multiple SENSE3-based algorithms like MUSE4 and POCS-ICE5 have been proposed to estimate and correct for the shot-specific phase errors. However, segmentation factors are still limited by the g-factor penalty and the time-consumption of iterative algorithms. This work investigates the possibility to recover corrupted multi-shot images using deep learning6 and exploits them for magnitude-regularized shot phase estimation7, thereby adapting the idea of synergistic machine learning and joint reconstruction8.Algorithms
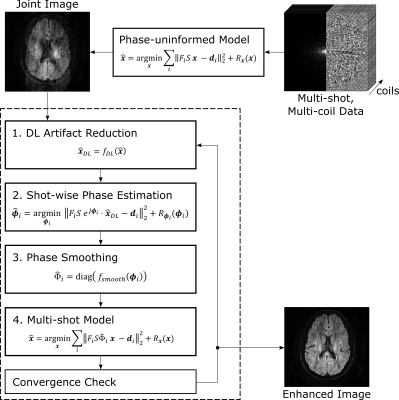
After an initialization, the proposed magnitude-regularized phase estimation (MAPE) algorithm iteratively repeats four steps. The initial joint image is estimated via standard SENSE reconstruction of the joint multi-shot k-space data thereby exhibiting phase-related signal cancellations. In the first step, a U-Net9 is applied on the corrupted joint image guess to mitigate the artifacts. Second, the U-Net corrected joint image is fixed for all shots and magnitude-regularized phase cycling7 is performed for each shot separately. Third, the resulting phases are smoothed. Finally, the extended SENSE10 problem is solved using the current shot phase estimates. Here, it should be noted that the final multi-shot model is strictly isolated from the neural network. The algorithm is summarized in Fig. 1.The proposed method without (MAPE) and with U-Net (MAPE + U-Net) is compared to extra-navigated IRIS10 and self-navigated iterative POCS-ICE5 reconstructions. Partial Fourier reconstructions are enabled by a homodyne approach, performing the multi-shot models on both low- and high-resolution data. Ten phase cycles were performed for each joint image update with db6 wavelet denoising. The smoothing was performed using a triangular window with half matrix size. Convergence was assumed when the residual error5 of subsequent iterations fell below 10-6 or the number of phase updates exceeded 200.
Methods
The U-Net9 was constructed using PyTorch, mapping magnitudes from input to output with size (240, 240). The encoder concatenates residual blocks11 (kernel size 3, ReLU activation, batch normalization) on five resolutions using max pooling for downsampling, while repeatedly doubling the channel number from 32 to 512. The decoder is constructed inversely using bilinear interpolation. The U-Net was trained for 100 epochs with batch size of 20 on a GeForce RTX 2080 Ti. The optimization was performed using ADAM12 (beta1 = 0.5, beta2 = 0.999) with a standard MSE loss. The learning rate was initially set to 10-4 and gradually reduced to 10-5 and 10-6 after every 40 iterations.In-vivo brain data with 4, 5, and 8 shots was attained from 7 healthy volunteers using a 13-channel head coil, 3T Philips Ingenia, b=1000 s/mm2 in three orthogonal orientations and 1x1x4 mm3 resolution. Informed consent was attained according to the rules of the institution. An extra-navigated DWI sequence10 was used to provide robust reference data for the learning task. Learning data was generated using the forward model with randomly scaled (zero-mean) in-vivo phase maps of the extra-navigated reconstructions. Train and test data were split on a subject basis (6/1). Moreover, 5-shot DTI datasets with 15 orientations were acquired for tensor analysis.
Results
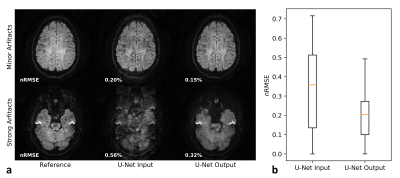
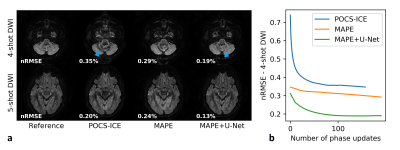
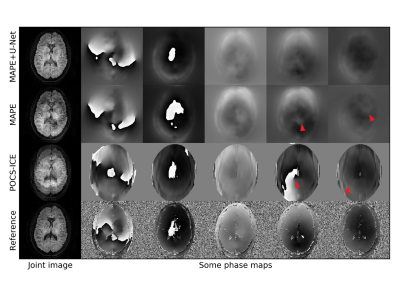
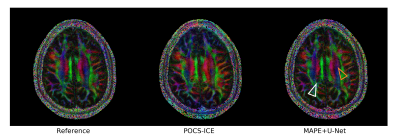
Figure 2 analyses the U-Net performance on the initial joint image. Figure 3 shows representative multi-shot reconstructions. Reconstructions of a highly segmented 8-shot datasets are summarized in Fig. 4. Figure 5 compares fractional anisotropy maps calculated using Dipy13 for the 5-shot DTI dataset. Normalized root-mean-square errors (nRMSE) were calculated with respect to the robust extra-navigated reconstruction. Note that this reference is not a proper ground truth, as it resulted from a navigated experiment and should be interpreted with care.Discussion
The neural network effectively mitigates signal cancellation artifacts at the cost of blurring, while leaving uncorrupted data unchanged (Fig. 2). The capability to fill the signal gaps proves very valuable to iteratively recover phase information from the data, especially in areas with high g-factor penalty and strong phase disturbances as in the brain base (Fig. 3). This joint image-constrained phase reconstruction pushes the segmentation limits (Fig. 4) and enables robust self-navigated reconstructions even for large DTI datasets (Fig. 5). Performance comparisons to related synergistic methods8 remain subject to further research.Conclusion
Magnitude-regularized phase estimation (MAPE) offers an effective iterative framework to improve the shot phase estimation in self-navigated multi-shot DWI by deep learning techniques, while keeping a conventional, interpretable joint image production.Acknowledgements
No acknowledgement found.References
1. Wu W and Miller KL. Image formation in diffusion MRI: A review of recent technical developments: Review of Image Formation in dMRI. JMRI. 2017;46(3):646–662.
2. Miller KL and Pauly JM. Nonlinear Phase Correction for Navigated Diffusion Imaging. MRM. 2003;50:343-353.
3. Pruessmann et al. SENSE: sensitivity encoding for fast MRI. MRM. vol. 1999;42(5):952–962.
4. Chen N, Guidon A, Chang H-C, Song AW. A robust multi-shot scan strategy for high-resolution diffusion weighted MRI enabled by multiplexed sensitivity-encoding (MUSE). NeuroImage. 2013;72:41-47.
5. Guo et al. POCS‐enhanced inherent correction of motion‐induced phase errors (POCS‐ICE) for high‐resolution multishot diffusion MRI. MRM. 2016;75(1):169-180
6. Haskell MW, Cauley SF, Bilgic B, et al. Network Accelerated Motion Estimation and Reduction (NAMER): Convolutional neural network guided retrospective motion correction using a separable motion model. 2019;82(4):1452-1461.
7. Ong F, Cheng J, Lustig M. General Phase Regularized Reconstruction using Phase Cycling. 2017. http://arxiv.org/abs/1709.05374. Accessed June 28, 2018.
8. Bilgic B, Chatnuntawech I, Manhard MK, et al. Highly accelerated multishot echo planar imaging through synergistic machine learning and joint reconstruction. MRM. 2019;82(4):1343-1358.
9. Jeong H-K, Gore JC, Anderson AW. High-resolution human diffusion tensor imaging using 2-D navigated multishot SENSE EPI at 7 T. MRM. 2013;69(3):793-802.
10. Ronneberger O, Fischer P, Brox T. U-net: Convolutional networks for biomedical image segmentation. In: International Conference on Medical Image Computing and Computer-Assisted Intervention. 2015:234–241.
11. Zhang K, Zuo W, Chen Y, Meng D, Zhang L. Beyond a Gaussian denoiser: residual learning of deep CNN for image denoising. IEEE Trans Image Process. 2017;26:3142–3155.
12. Diederik P. Kingma and Jimmy Lei Ba. Adam: a Method for Stochastic Optimization. International Conference on Learning Representations. 2015:1-13.
13. Garyfallidis E, Brett M, Amirbekian B, et al. Dipy, a library for the analysis of diffusion MRI data. Frontiers in neuroinformatics. 2014;8:8.
Figures