4363
Towards the clinical application of model‐based reconstruction framework for distortion correction in prostate diffusion weighted MRI.1Centre for Medical Imaging, University College London, London, United Kingdom, 2Department of Radiology, University College Hospital, London, United Kingdom
Synopsis
Prostate diffusion-weighted (DW) MRI based on single-shot echo planar imaging (EPI) can suffer from geometric distortions caused by off-resonance magnetic field at the prostate-rectal air interface. Although techniques exist for effective distortion correction in brain imaging, such approaches may struggle with the severe distortions observed in prostate imaging. Here we focus on applying a recently developed model-based reconstruction technique to correct distortions in typical clinically used prostate DW-MRI. The distortion correction is feasible and may improve prostatic zonal anatomy in DW-MRI. Additionally, corrected averaged high b-value datasets show higher SNR than uncorrected dataset and may further help to identify tumours.
Introduction
Prostate diffusion-weighted (DW) MRI are used as part of multi-parametric (mp) MRI for detecting tumours. DW-MRI, based on single-shot echo planar imaging (EPI), can suffer from geometric distortions caused by off-resonant magnetic fields arising from susceptibility differences between prostate tissue and rectal air. Previously, Usman et al1,2 introduced two model-based reconstruction approaches for correcting distortions, including signal pile-ups, and were demonstrated on low resolution DW data with b-values of 0 and 500s/mm2 only. The correction process requires two opposite phase-encoded DW-EPI datasets with equal number of measurements and a B0-field map. The aim of this work is to demonstrate the application of one of the model-based reconstruction approaches1 on typical clinical prostate DW-MR datasets.Methods
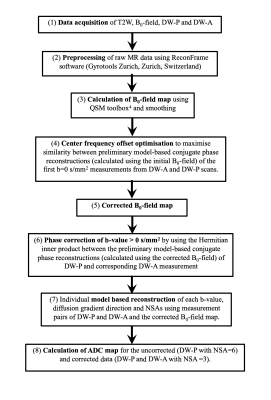
Image acquisition: Two prostate MR patients (60-70y.o.) consented for additional acquisitions. Four MR scans (T2-weighted (T2W), B0-field and two DW scans) were acquired within 17minutes. (1)An axial T2W image with turbo spin echo, resolution=0.6×0.6×3mm3, field of view (FOV)=220×220×57mm3, SENSE=1.3, TE/TR=100msec/4000msec. (2)A B0-field map was calculated using a 3D dual-echo gradient-echo scan with resolution=1.3×1.3×1.3mm3, FOV=230×230×92mm3, partial-Fourier acquisition=0.78 (slice direction), TE/TR=4msec/9msec. (3)A typical clinical prostate DW-MR protocol with single-shot EPI, resolution=1.3×1.3×5mm3, FOV=220×220×55mm3, partial-Fourier acquisition=0.63, SENSE=1.6, phase‐encode bandwidth/pixel=42.6Hz/pixel, TE/TR=80msec/2600msec, b-values of 0s/mm2 (Number of signal averages (NSA)=6), 150s/mm2 (NSA=6), 500s/mm2 (NSA=12) and 1000s/mm2 (NSA=18) each with three orthogonal diffusion directions and phase‐encoding direction in the anterior‐posterior (AP) direction (DW-P). Only half of the NSAs from this DW-P dataset were used for model-based reconstruction. (4)The same DW sequence but with opposite phase-encoding direction (DW-A) and half of the NSAs of DW-P was also acquired for model-based reconstruction. All sequences used first order volume shimming on the prostate region and its vicinity.Data post-processing: Data was processed similarly to1 and is illustrated in Fig.1. However, some modifications were made to the pipeline. The large raw EPI datasets (approx.11GB per patient) were reduced 4x in size using array coil compression3 to enable preprocessing on a MacbookPro (16GB RAM). Information about the shim gradients was extracted from the DICOM files and the B0-field maps were modified to account for differences in shim gradient induced B0-fields between DW and B0 scans.
To compensate for frequency changes between the DW-P and DW-A scans, a reliable fixed center frequency offset was computed using an iterative optimisation process with modified objective function and multiple start points (ranging from -40 to 40Hz). Phase correction for the DW datasets with b-values >0s/mm2 were carried out to minimise SNR loss during averaging. Due to the high-resolution data with many b-values and NSAs, model-based reconstruction was performed using University College London’s research computing platform, where correction of each individual measurement (DW-P and DW-A pair) using 16GB RAM and 12 parallel processors took approx.15 minutes.
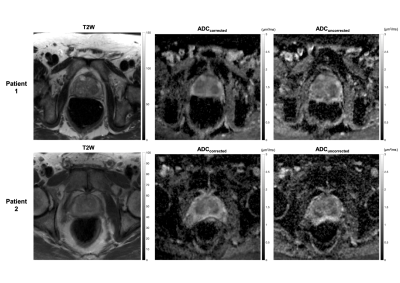
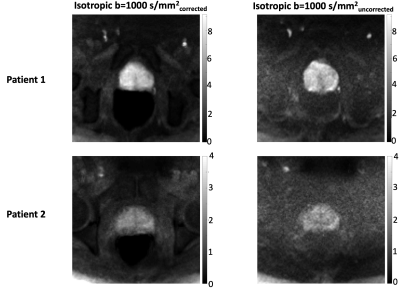
Image analysis: Uncorrected ADC maps (using DW-P NSA=6) and corrected ADC maps (using DW-P NSA=3 and DW-A NSA=3) were calculated for all slices of the prostates and qualitatively compared to the T2W images resampled to the same resolution and slice thickness. Prostate ROIs were drawn on the T2W images, uncorrected and corrected ADC datasets. The Dice similarity scores with respect to the T2W images were calculated. The uncorrected and corrected isotropic b=1000s/mm2 were compared visually with the T2W image and the signal to noise ratio (SNR) at the prostate was calculated using the difference method5.
Results
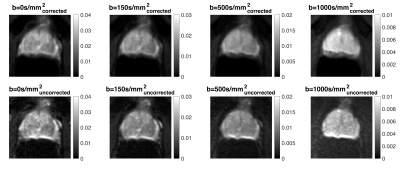
Fig.2 demonstrates that the corrected ADC maps have higher Dice scores and prostatic zone similarity to T2W images than the uncorrected ADC maps. Fig.3 shows that corrected isotropic b=1000s/mm2 have higher SNR (7.2±1.3 and 3.0±0.3) than uncorrected data (3.9±0.5 and 2.1±0.4) suggesting the phase correction step is effective. However, some blurring is observed in the corrected b=1000s/mm2 data. A further comparison (Fig.4) of all b-values for a different corrected slice of Patient 1 shows visibly increasing shifts in the prostate in the phase-encoding direction with increasing b-values, which suggests scanner frequency drifts and potential contribution from eddy currents.Discussion
Early data indicates that distortion correction using model-based reconstruction for clinical standard dataset is challenging but feasible. The benefits are distortion correction including pile-up correction, improved DICE score (useful for automatic processing of mp-MRI) and significant increases in SNR of high b-value DW data - all for an extra scan time of 3minutes (the B0 scan). However, clinical data are usually large leading to lengthy postprocessing times. Another limitation is the static center frequency offset computed using the first b=0s/mm2 from DW-P and DW-A. Further frequency drifts are not currently considered for b-values that are acquired up to 4minutes after the initial b=0s/mm2 and may explain the blurring observed in Fig.4. Possible additions of extra b=0s/mm2 measurements at intervals, accounting for eddy currents and image acquisition using interleaved double spin-echo sequence6 may provide more accurate B0-field maps to prevent blurring. Pile-up correction is not always effective and future work aims to investigate the gradient of the B0 map, the B0 map smoothing applied and methods such as2 for refining the B0 map.Conclusion
Distortion correction of prostate DW-MRI with typical clinical scan parameters using model-based reconstruction is feasible but challenging. Potential one-to-one mapping of ADC maps to T2W images may improve mp-MRI but tighter control of B0 fluctuations and possibly eddy current effects may be required.Acknowledgements
This work is supported by Cancer Research UK/EPSRC (grant A21099) and the NIHR Biomedical Research Unit at UCH. We acknowledge research support provided by Philips Healthcare. The authors would also like to thank Elizabeth Isaac for recruiting the patients.References
[1] Usman, M, Kakkar, L, Kirkham, A, Arridge, S, Atkinson, D. Model‐based reconstruction framework for correction of signal pile‐up and geometric distortions in prostate diffusion MRI. Magn Reson Med. 2019;81:1979-1992
[2] Usman, M, Kakkar, L, Matakos, A, Kirkham, A, Arridge, S, Atkinson, D. Joint B0 and image estimation integrated with model based reconstruction for field map update and distortion correction in prostate diffusion MRI. Magn Resonance Imag. 2019:65:90-99.
[3] Buehrer, M. , Pruessmann, K. P., Boesiger, P. and Kozerke, S. Array compression for MRI with large coil arrays. Magn. Reson. Med. 2007:57:1131-1139
[4] Quantitative Susceptibility Mapping Toolbox. Cornell MRI Research Laboratory. http://weill.cornell.edu/mri/pages/qsm. html. Accessed April 28, 2018.
[5] Dietrich, O. , Raya, J. G., Reeder, S. B., Reiser, M. F. and Schoenberg, S. O. Measurement of signal‐to‐noise ratios in MR images: Influence of multichannel coils, parallel imaging, and reconstruction filters. J. Magn. Reson. Imaging, 2007:26:375-385.
[6] Hutter, J, Christiaens, D, Deprez, M, Cordero-Grande, L, Slator, P, Price, A, Rutherford, M, Hajnal, J. Dynamic Field Mapping and Motion Correction Using Interleaved Double Spin-Echo Diffusion MRI. MICCAI 2017:523-531
Figures