4354
Interleaved Block-Segmented Echo-Planar Imaging (iblocks-EPI) based Diffusion-Tensor Imaging with Retrospective Motion Correction1Department of Diagnostic Radiology, The University of Hong Kong, Hong Kong, China, 2Graduate Institute of Biomedical Electronics and Bioinformatics, National Taiwan University, Taipei, Taiwan, 3Department of Biomedical Engineering, University of Arizona, Tucson, AZ, United States, 4Brain Imaging and Analysis Center, Duke University Medical Center, Durham, NC, United States
Synopsis
Recently, a self-navigated interleaved block-segmented EPI (iblocks-EPI) has been proposed to acquire DTI data with high spatial resolution and less geometric diction. In addition, the oversampling of central k-space of iblock-EPI can benefit the SNR performance. However, same as the other multi-shot EPI techniques, iblocks-EPI is highly susceptible to minuscule and macroscopic motions during data acquisition. In this study, we developed a self-calibrated and collaborative iblocks-DTI reconstruction framework that can correct image artifacts and diffusion-encoding contrast change caused by minuscule and macroscopic motions.
Purpose
Diffusion tensor imaging (DTI) is an important tool for investigating tissue micro-structure and mapping white matter fibers in human brain.1–3 The use of single-shot EPI for DTI acquisition can only achieve limited spatial resolution and low geometric fidelity. Recently, a self-navigated interleaved block-segmented EPI4 (iblocks-EPI) has been proposed to acquire DTI data with high spatial resolution and less geometric diction. In addition, the oversampling of central k-space of iblock-EPI can benefit the SNR performance. However, same as the other multi-shot EPI techniques, iblocks-EPI is highly susceptible to two types of motions during data acquisition.5 First, the minuscule motion (e.g., brain pulsation) can induce inter-shot phase variations, leading to aliasing artifact after combining all segment data together. Second, macroscopic motion (e.g., head movement) can cause image blurring and inaccurate diffusion tensor estimations in reconstructed image6. Therefore, POCSMUSE7and AMUSE have been proposed to correct the motion-induced problems during interleaved EPI acquisition. In this study, we developed a self-calibrated and collaborative iblocks-DTI reconstruction framework that can correct image artifacts and diffusion-encoding contrast change caused by minuscule and macroscopic motions.Material & Method
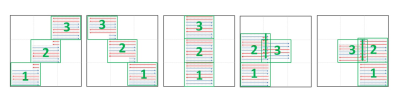
iblocks-EPI acquisition scheme:A 4-shot interleaved EPI sequence was modified to acquire data in five different patch patterns, and each patch consists of three k-space blocks that were acquired with 4 ky-segments. Figure 1 shows the design of patch pattern for proposed iblocks-EPI acquisition.
Reconstruction framework:
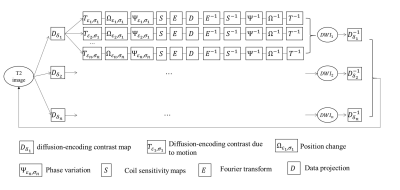
The k-space signal acquired from ε-th ky-segment of σ-th patch can be presented as:
Kε,σ = Mσ E Sγ Ψε,σΩε,σTε,σDδp0
where p0 represents the un-aliased image, Dδ the contrast map of δ-th diffusion-encoding direction,Tε,σ , Ψε,σ and Ωε,σ the diffusion-encoding contrast change caused by rotation, phase variations and position changes of the image acquired from ε-th ky-segment and σ-th patch, Sγ the coil sensitivity maps of γ-th coil, E the Fourier encoding matrix, and Mσ the sampling mode of σ-th patch.
First, POCSENSE8 was used to reconstruct images for each individual ky-segment data for all patches and diffusion direction. Afterward, the phase variation maps Ψ, matrix of subject motions Ω, and diffusion-encoding contrast maps T of each individual ky-segment were estimated from POCSENSE produced images. Second, a POCSMUSE-based framework was used to jointly reconstruct all diffusion-encoded images from all ky-segment data of all diffusion directions.
Figure 2 shows the proposed reconstruction framework as follow: 1) Starting from an initial guess of T2 image, the diffusion-encoded images were generated by applying diffusion-encoding contrast maps; 2) Image of each shot was generated by applying specific motion-induced diffusion contrast map, spatial transformations, phase variations, and coil sensitivity map; 3) Data were updated by replacing generated k-space data with experimentally acquired data; 4) updated data from all diffusion directions were combined to generate an updated T2 image, which was used as the input image for next iteration.
Experiments:
Human brain DTI data (b=0 and b=750s/mm2) with 15 DTI directions were acquired using a 1.5T MRI scanner (Lift Explorer 1.5T MRI, General Electric, USA) with an 8-channel coil (TE/TR = 84/4000ms, FOV = 24cm, matrix size = 192x192). Two sets of data were obtained from one volunteer with two different head positions (i.e., different in-plane rotations). After acquisition, these two datasets were combined to create a synthetic k-space dataset corrupted by both minuscule (phase errors) and macroscopic (head rotation) motions.
Five algorithms were tested for image reconstruction of iblocks-EPI based DTI acquisition:
a. No motion correction: Images were produced by directly applying 2D Fourier transform.
b. Minuscule motion correction using POCSMUSE: Only phase errors caused by minuscule motion were corrected.
c. Minuscule and macroscopic motion correction using POCSMUSE (ie. POCS-AMUSE*): Phase errors caused by minuscule motion and position rotations were simultaneously corrected.
d. Minuscule and macroscopic motion correction with diffusion-encoding contrast correction using POCSMUSE (ie. POCS-AMUSE): The change in diffusion-encoding contrast due to macroscopic rotation was also corrected during data reconstruction.
e. Minuscule and macroscopic motion correction with diffusion-encoding contrast correction using our proposed framework (Figure 2): All diffusion-encoded images were reconstructed jointly.
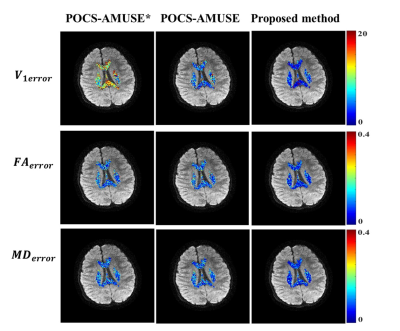
Only the results produced from last three methods (c-e) were used for tensor calculation. Three quantitative criteria were used to assess their tensor calculation accuracy: 1) the percentage error in fractional anisotropy (FAerror); 2) the percentage error in mean diffusivity (MDerror); and 3) the angular deviation of the principal eigenvectors (V1error).
Result
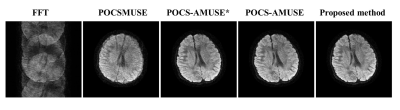
Figure 3 shows representative diffusion-encoded images reconstructed with five different methods. Severe artifacts appear in the image with direct Fourier transform. POCSMUSE can effectively suppress aliasing artifacts, but it is not capable of eliminating image blurring caused by macroscopic motion. POCS-AMUSE*, POCS-AMUSE and our proposed method all can produce diffusion-encoded images without motion-induced artifacts. Figure 4 shows the error maps of tensor estimation for three different reconstruction methods.Discussion
Since POCS-AMUSE* does not consider the diffusion contrast changes caused by macroscopic motion, its calculated v1 maps shows larger deviations compared with gold standard than the other two methods. In this preliminary evaluation, our proposed method shows greater reductions in all error maps compared with POCS-AMUSE. In conclusion, the proposed framework can effectively eliminate the artifacts caused by motions in iblocks-EPI based DTI, and can provide more accurate diffusion tensor information than other tested methods.Acknowledgements
The work was in part supported by grants from Hong Kong Research Grant Council (GRF HKU17121517) and Hong Kong Innovation and Technology Commission (ITS/403/18).References
1. Le Bihan D, Van Zijl P. From the diffusion coefficient to the diffusion tensor. NMR Biomed. 2002;15(7-8):431-434. doi:10.1002/nbm.798
2. Le Bihan D. Looking into the functional architecture of the brain with diffusion MRI. Int Congr Ser. 2006;1290(June):1-24. doi:10.1016/j.ics.2006.04.006
3. Mori S, Van Zijl PCM. Fiber tracking: Principles and strategies - A technical review. NMR Biomed. 2002;15(7-8):468-480. doi:10.1002/nbm.781
4. High-quality and self-navigated diffusion-weighted imaging enabled by a novel interleaved block-segmented (. 2014:5898.
5. Wu W, Miller KL. Image formation in diffusion MRI: A review of recent technical developments. J Magn Reson Imaging. 2017;46(3):646-662. doi:10.1002/jmri.25664
6. Guhaniyogi S, Chu ML, Chang HC, Song AW, Chen NK. Motion immune diffusion imaging using augmented MUSE for high-resolution multi-shot EPI. Magn Reson Med. 2016;75(2):639-652. doi:10.1002/mrm.25624
7. Chu ML, Chang HC, Chung HW, Truong TK, Bashir MR, Chen NK. POCS-based reconstruction of multiplexed sensitivity encoded MRI (POCSMUSE): A general algorithm for reducing motion-related artifacts. Magn Reson Med. 2015;74(5):1336-1348. doi:10.1002/mrm.25527
8. Samsonov AA, Kholmovski EG, Parker DL, Johnson CR. POCSENSE: POCS-based reconstruction for sensitivity encoded magnetic resonance imaging. Magn Reson Med. 2004;52(6):1397-1406. doi:10.1002/mrm.20285
Figures