4351
Deep learning-based partial Fourier reconstruction for improved prostate DWI
Fasil Gadjimuradov1,2, Seung Su Yoon1,2, Thomas Benkert2, Marcel Dominik Nickel2, Karl Engelhard3, and Andreas Maier1
1Pattern Recognition Lab, Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, Germany, 2Magnetic Resonance, Siemens Healthcare GmbH, Erlangen, Germany, 3Department of Radiology, Martha-Maria Hospital, Nürnberg, Germany
1Pattern Recognition Lab, Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, Germany, 2Magnetic Resonance, Siemens Healthcare GmbH, Erlangen, Germany, 3Department of Radiology, Martha-Maria Hospital, Nürnberg, Germany
Synopsis
Partial Fourier (PF) acquisition schemes are a common way to increase the inherently low signal-to-noise ratio in diffusion-weighted (DW) images. The naïve solution of zero-filling k-space results in visible blurring and Gibbs ringing. Based on the circumstance that traditional methods such as homodyne reconstruction or POCS often fail to remove blurring and ringing without introducing new artifacts, this work aims to use a Convolutional Neural Network for robust PF reconstruction in prostate DWI. We show that our data-driven approach, which efficiently uses correlations across different b-values, outperforms traditional methods in terms of quantitative measures and visual impression of the images.
Introduction
Diffusion weighted imaging (DWI) has emerged as a valuable tool for the assessment of prostate cancer, providing high sensitivity in lesion detection1. However, prostate DWI inherently suffers from low signal-to-noise ratio (SNR). When using EPI sequences, partial Fourier (PF) sampling along the phase-encoding direction shortens the echo train and thereby the effective TE, resulting in increased signal.Zero-filling (ZF) the missing k-space lines inevitably leads to blurring and Gibbs ringing in the reconstructed image. Commonly used techniques for PF reconstruction, such as Projection onto Convex Sets2 (POCS) and homodyne reconstruction3 produce unsatisfactory results in regions with rapid phase variations and tend to introduce additional noise when estimating high-frequency components. Alternatively, Convolutional Neural Networks (CNNs) have been proposed for PF reconstruction, showing promising results for Dixon imaging4 and brain DWI5. However, it remains unclear how these approaches generalize to the task of PF reconstruction in prostate DWI which is particularly difficult due to low SNR.
The goal of this work is to develop a CNN-based method for PF reconstruction of DW images of the prostate. In contrast to previous work, correlations across different b-values are exploited.
Methods
Data: A prototype EPI sequence was used to acquire fully-sampled DW images of the prostate at two b-values (50 and 800$$$\,$$$s/mm2) in 18 male volunteers on 1.5$$$\,$$$T and 3$$$\,$$$T MR scanners (MAGNETOM, Siemens Healthcare GmbH, Erlangen, Germany). In addition, one patient with suspected prostate cancer was scanned to showcase the clinical value. While 15 volunteer data sets were used for training, the data of one volunteer served as a validation set. The remaining two volunteers as well as the patient data set were used for testing. Instead of operating on the combined, trace-weighted magnitude images, our model was applied to the complex-valued images of the single shots. In order to augment the data set, images were acquired with two different phase-encoding directions (RL and AP) and with both single-shot EPI and reduced FOV EPI (ZOOMit). In total, the training, validation and test splits contained 10944, 768 and 1536 images, respectively.CNN training: For preprocessing, images were retrospectively downsampled by multiplication with a PF mask in k-space, yielding the zero-filled images. For training, the images were interpolated to a fixed resolution of 100x100 pixels. The complex-valued images were presented as a two-channel input to the network. A modified version of a state-of-the-art CNN in the related field of super-resolution was used as network architecture6 (see Figure 1 for details). Training was performed by minimizing the L1-loss between the network output and the fully-sampled ground-truth images using the Adam optimizer7 with a learning rate of 10-4. Based on the observation that the deblurring ability of the network on low-signal b800 images was poor, we tried to utilize the correlations between different b-values by concatenating b50 and b800 images along the channel dimension. Although b50 and b800 images show different contrasts, they share similar structural information, hence, allowing the less noisy b50 image to serve as a "guidance". As a post-processing step which further enforced data fidelity, the k-space of the output images was substituted by measured data where it was available.
Evaluation: The results on the test set were evaluated quantitatively and qualitatively and compared to standard PF reconstruction methods. In addition to retrospectively and prospectively undersampled trace-weighted images, the performance was analyzed on derived ADC maps as well.
Results & Discussion
Table 1 gives an overview of the quantitative results of our CNN and the reference methods zero-filling (ZF), POCS and homodyne reconstruction. On the entire test set, our CNN outperforms the next-best method (POCS) by +$$$\,$$$0.0175 (SSIM) and +$$$\,$$$1.45$$$\,$$$dB (PSNR). For b800 images, the improvements are lower than for b50 images, underlining the difficulty of training the network for inputs with very low SNR.The qualitative comparison on retrospectively undersampled images presented in Figure 2 confirms the quantitative results. While the ZF reconstruction suffers from strong blurring and ringing, POCS as well as the homodyne reconstruction are capable of restoring resolution. However, this comes at the cost of a structured noise pattern across the image. In contrast, our method performs deringing and deblurring without visual noise enhancement. The above-mentioned observations equivalently hold true for prospectively undersampled images (see Figure 3).
Figure 4 shows the ADC map of a patient with suspected prostate cancer. While an area of restricted diffusion is clearly delineated in the fully-sampled ground-truth image, blurring in the ZF reconstruction makes it more difficult to differentiate it from normal tissue. Our CNN-based method restores image quality and sharpness nearly to the level of the ground-truth.
Conclusion
We showed that a CNN-based reconstruction enables robust PF imaging. Unlike traditional reconstruction techniques, our method recovers image quality without introducing noticeable artifacts. Furthermore, correlations across b-values are exploited to improve performance for high b-values. The proposed technique provided promising results for prostate DWI in healthy volunteers and one patient. Although it was only applied to an extreme case of a PF factor of 5/8 in this work, the concept is similarly applicable to other commonly used PF factors. A clinical validation is the subject of future work.Acknowledgements
No acknowledgement found.References
- Turkbey B, Rosenkrantz AB, Haider MA, et al. "Prostate Imaging Reporting and Data System Version 2.1: 2019 Update of Prostate Imaging Reporting and Data System Version 2." European Urology (2019).
- Haacke EM, Lindskogj ED, and Lin W. "A fast, iterative, partial-Fourier technique capable of local phase recovery." Journal of Magnetic Resonance, 92(1):126-145 (1991).
- Noll DC, Nishimura DG, and Macovski A. "Homodyne detection in magnetic resonance imaging." IEEE transactions on medical imaging, 10(2):154-163 (1991).
- Toews AR,
Alley MT, Vasanawala SS, et al. "Deep Partial Fourier Reconstruction."
Proceedings of the ISMRM, #48702 (2019).
- Muckley MJ,
Ades-Aron B, Papaioannou A, et al. "Training a Neural Network for Gibbs
and Noise Removal in Diffusion MRI." http://arxiv.org/abs/1905.04176
(2019). Accessed
November 5, 2019.
- Zhang Y, Tian Y, Kong Y, et al. "Residual Dense Network for Image Super-Resolution." Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, 2472-2481 (2018).
- Kingma DP, and Ba J. "Adam: A method for stochastic optimization." http://arxiv.org/abs/1412.6980 (2014). Accessed November 5, 2019.
Figures
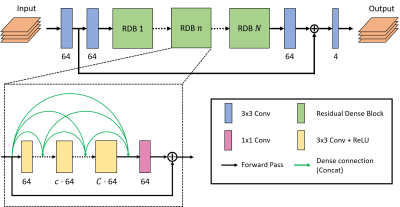
Figure 1: Modified
version of the Residual Dense Network6 (RDN) architecture where $$$N$$$ is the number of Residual Dense
Blocks (RDBs) and $$$C$$$ is the number of convolutional layers (Convs) in every RDB.
In our approach, $$$N=8$$$ and $$$C=4$$$ were chosen.
The
number of output channels
is displayed below every Conv. Inside RDBs,
convolutional layers are densely connected among each other, allowing to use
feature information extracted at earlier layers as well. The network input and output
have 4 channels each (b50real, b50imag, b800real, b800imag).
Table 1: Quantitative comparison of PF reconstruction
performance of different methods. The mean structural similarity
(SSIM) and mean peak signal-to-noise ratio (PSNR) on the test data set are
shown. Best and second-best results are highlighted in red and blue,
respectively.
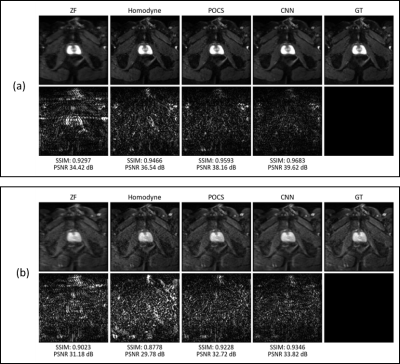
Figure 2: Qualitative comparison of retrospectively
undersampled, trace-weighted images for b-values 50 (a) and 800$$$\,$$$s/mm2 (b).
The top row shows the image reconstructed by each method while the bottom row
displays the residual to the ground-truth (GT) image multiplied by a factor of
10. While the ZF image exhibits blurring in RL direction, the POCS and homodyne
reconstructions introduce noise and leave some ringing in the image. The
output of our CNN-based method has noise properties comparable to the
ground-truth and yields an overall smaller and less structured residual.
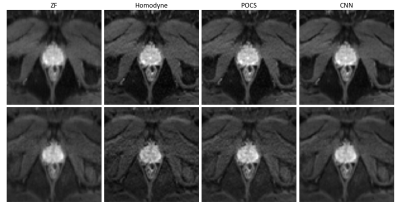
Figure 3: Qualitative
comparison of prospectively
undersampled, trace-weighted images for b-values (top row) and 800$$$\,$$$s/mm2 (bottom row). Again, the ZF
reconstruction shows excessive blurring (along the AP direction in this case) which can be minimized by all of the other
methods. However, the POCS and homodyne reconstructions add noise to the images
which can be avoided using the CNN-based method.
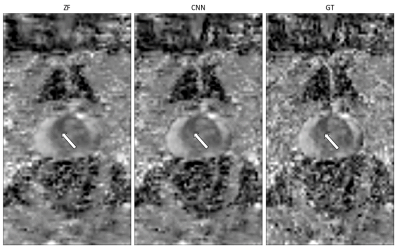
Figure 4:
Qualitative comparison of ADC maps of a patient with suspected prostate
cancer. While an area of restricted diffusion (indicated by the white arrow) is
blurred in the RL direction in the ADC map of the ZF reconstruction, its borders
in the CNN reconstruction are almost as well-defined as in the ground-truth.