4342
High-resolution distortion-free single-shot EPI enabled by deep-learning1Center for Biomedical Imaging Research, Department of Biomedical Engineering, School of Medicine, Tsinghua University, Beijing, China, 2China National Research Center for Neurological Diseases, Beijing Tiantan Hospital, Capital Medical University, Beijing, China, 3Philips Healthcare, Beijing, China, 4MR Clinical Science, Philips Healthcare (Suzhou), Suzhou, China
Synopsis
Single-shot EPI (SS-EPI) is widely used for diffusion-weighted imaging (DWI), but suffers from susceptibility-induced distortion and T2* blurring, which limit its resolution and ability to detect detailed structures. Parallel imaging and multi-shot techniques can be used to improve the resolution and reduce image distortion. However, these techniques have their own drawbacks, such as limited achievable acceleration factors or prolonged acquisition time. In this study, a deep-learning based method is proposed to achieve high-resolution distortion-free DWI using SS-EPI thus to improve the acquisition efficiency and clinical applicability.
Introduction
Echo-planar imaging (EPI), especially single-shot EPI has been widely used for diffusion-weighted imaging (DWI), but suffers from susceptibility-induced distortion and T2* blurring, which limits its resolution and the ability to detect detailed structures. Parallel imaging techniques 1,2 have been applied to SS-EPI to improve the resolution and reduce image distortion, which however is limited by the achievable parallel imaging acceleration factor. As an alternate, a number of multi-shot EPI approaches have been proposed to reduce the distortion while maintaining high SNR 3-7, among which point-spread function (PSF) encoded EPI (PSF-EPI) can achieve totally distortion-free imaging. Nevertheless, no matter which multi-shot technique, to achieve high-resolution imaging with very low or no distortion, a large number of shots is required, which means severely prolonged acquisition time and limited clinical application. Recently, deep-learning using a neural network has been applied across multiple MR imaging fields, such as reconstruction, denoising, and contrast synthesis 8-10. In this study, the SS-EPI images were used as the input and PSF-PEI images were used as the labels to train the neural network, thus to achieve high-resolution distortion-free DWI while maintaining the time efficiency of conventional SS-EPI.Methods
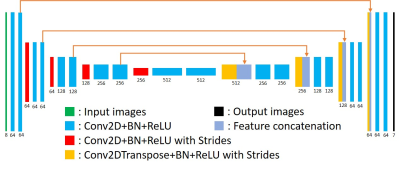
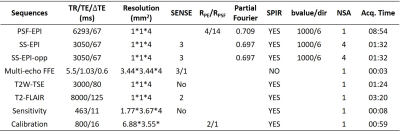
(1) Data acquisition: Detailed data acquisition protocol can be found in Table. 1, in which images of different contrasts were acquired for the brain. SS-EPI and PSF-EPI DWI were acquired with 1×1 mm2 in-plane resolution. SS-EPI with opposite phase encoding (PE) direction (SS-EPI-opp) was acquired for the top-up distortion correction 11. B0 maps acquired by multi-echo gradient echo were obtained for the distortion correction of SS-EPI using the field-map method 12. PSF-EPI DWI was acquired with 4-fold acceleration along the PE direction and 14-fold acceleration along the PSF encoding direction. Additional sensitivity and calibration data were acquired for the reconstruction of PSF-EPI 7. The matrix size of SS-EPI was 217×220, and for PSF-EPI, it was 221×220. T2W-TSE and T2W-FLAIR-TSE were also acquired. All images were acquired using FOV = 220×220×100 mm3 with 25 axial slices covering the whole brain. Ten healthy volunteers provided with written informed consent were scanned on a Philips 3T scanner (Philips Healthcare, Best, The Netherlands).(2) Network architecture: The network architecture of the proposed method is shown in Fig. 1, in which a 2D U-net was used as the base structure13. The network consisted of 19 convolutional layers, 4 convolutional layers with strides for downsampling, 4 deconvolutional layers with strides for upsampling, and 4 feature contracting paths. Batch-normalization (BN) and ReLU were used for each layer. To improve the output image quality, T2W-TSE images, which are commonly used as a routine clinical MR examination, were also fed into the network to provide additional structural information. Thus the input layer consisted of 8 channels (including 1 b = 0 s/mm2 image and 6 b = 1000 s/mm2 images and 1 T2W-TSE image), the output image consisted of 7 channels (including 1 b = 0 s/mm2 image and 6 b = 1000 s/mm2 images).
(3) Training and evaluation: Images from 7 volunteers were used for training, images from 2 volunteers were used as a validation set, and images from 1 volunteer were used as a test set. Data were augmented through patching along the FE direction since distortion only occurs along the PE direction. The patch size was 32, thus the input dimension of the network was 217×32×8, and the output dimension was 221×32×7. The network was trained and evaluated using Keras 14. Moreover, clinical SS-EPI images acquired with different parameters (in figure caption) on 2 stroke patients (IRB approved) on different platforms (Siemens and GE 3T scanners) were fed into the network trained using the acquired healthy volunteers’ data after interpolation to match the input dimension of the network to test the generalization ability of the proposed method.
Results and Discussion
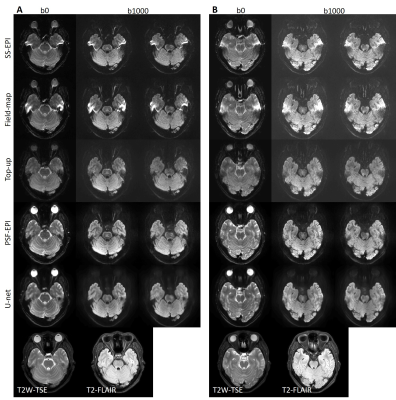
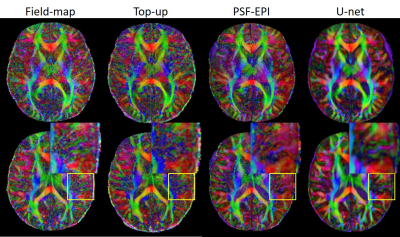
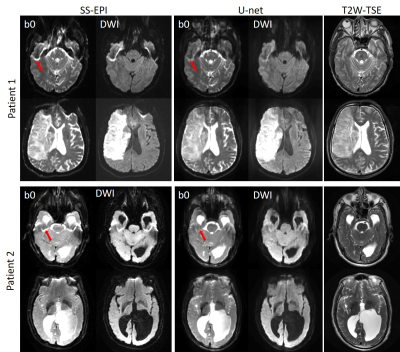
Fig. 2 shows the results of the proposed method (denoted by U-net), which are compared with the original SS-EPI images, the SS-EPI images after distortion correction using the field-map method (denoted by field-map), the SS-EPI images after distortion correction using the top-up method (denoted by top-up), and the PSF-EPI images. The results indicate that the proposed method can correct the distortion much better than the traditional methods and can improve the SNR. Fig. 3 shows the FA maps calculated using the corrected images from U-net, which were compared with the FA maps calculated using the results from field-map, top-up and PSF-EPI images, respectively. The results show that the improved SNR can help with the delineation of detailed structures. Fig. 4 shows the applications of the proposed method on patients’ data. The network trained on the healthy volunteers worked well and can correct the distortion effectively, which indicate the good generalization ability of the proposed method.Conclusion
The proposed method can achieve high-resolution distortion-free DWI using SS-EPI, thus can maintain the time efficiency compared with the traditional multi-shot techniques. Meanwhile, the network can boost the SNR of the output images to help with the delineation of detailed structures. In conclusion, the proposed method has the potential to improve the clinical applicability of high-resolution DWI.Acknowledgements
No acknowledgement found.References
1. Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: Sensitivity encoding for fast MRI. Magnetic resonance in medicine 1999;42(5):952-962.
2. Griswold MA, Jakob PM, Heidemann RM, et al. Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magnetic resonance in medicine 2002;47(6):1202-1210.
3. Butts K, de Crespigny A, Pauly JM, Moseley M. Diffusion-weighted interleaved echo-planar imaging with a pair of orthogonal navigator echoes. Magnetic resonance in medicine 1996;35(5):763-770.
4. Bammer R, Stollberger R, Augustin M, et al. Diffusion-weighted imaging with navigated interleaved echo-planar imaging and a conventional gradient system. Radiology 1999;211(3):799-806.
5. Holdsworth SJ, Skare S, Newbould RD, Guzmann R, Blevins NH, Bammer R. Readout-segmented EPI for rapid high resolution diffusion imaging at 3 T. European journal of radiology 2008;65(1):36-46.
6. In MH, Posnansky O, Speck O. High-resolution distortion-free diffusion imaging using hybrid spin-warp and echo-planar PSF-encoding approach. NeuroImage 2017;148:20-30.
7. Dong Z, Wang F, Reese TG, et al. Tilted-CAIPI for highly accelerated distortion-free EPI with point spread function (PSF) encoding. Magnetic resonance in medicine 2018.
8. Yoon J, Gong E, Chatnuntawech I, et al. Quantitative Susceptibility Mapping using Deep Neural Network: QSMnet. arXiv preprint arXiv:180305627 2018.
9. Gong E, Pauly JM, Wintermark M, Zaharchuk G. Deep learning enables reduced gadolinium dose for contrast-enhanced brain MRI. J Magn Reson Imaging 2018;48(2):330-340.
10. Zhu B, Liu JZ, Cauley SF, Rosen BR, Rosen MS. Image reconstruction by domain-transform manifold learning. Nature 2018;555(7697):487-492.
11. Andersson JLR, Skare S, Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. NeuroImage 2003;20(2):870-888.
12. Jezzard P, Balaban RS. Correction for geometric distortion in echo planar images from B0 field variations. Magnetic resonance in medicine 1995;34(1):65-73.
13. Ronneberger O, Fischer P, Brox T. U-net: Convolutional networks for biomedical image segmentation. 2015. Springer. p 234-241.
14. Chollet, François. Keras (https://github.com/fchollet/keras). GitHub repository 2015.
Figures