4338
DTI in early RRMS patients with correlation to clinical parameters and comparison to Healthy Controls
Abdulaziz Alshehri1,2, Oun Al-iedani1,2, Jameen Arm1,2, Neda Gholizadeh1, Rodney Lea3, Jeannette Lechner-Scott3,4,5, and Saadallah Ramadan1,2
1School of Health Sciences, University of Newcastle, Newcastle, Australia, 2Imaging center, Hunter Medical Research Institute, Newcastle, Australia, 3Hunter Medical Research Institute, Newcastle, Australia, 4School of Medicine and Public Health, University of Newcastle, Newcastle, Australia, 5Department of Neurology, John Hunter Hospital, Newcastle, Australia
1School of Health Sciences, University of Newcastle, Newcastle, Australia, 2Imaging center, Hunter Medical Research Institute, Newcastle, Australia, 3Hunter Medical Research Institute, Newcastle, Australia, 4School of Medicine and Public Health, University of Newcastle, Newcastle, Australia, 5Department of Neurology, John Hunter Hospital, Newcastle, Australia
Synopsis
This study aims to evaluate and compare DTI parameters in relapsing-remitting MS patients with age and sex-matched healthy controls, and to correlate these DTI metrics with clinical symptoms and brain volumetric measures. As a result, There was a statistically significant increase in most of DTI parameters for RRMS patients compared with healthy controls. FA correlated positively with clinical parameters like EDSS and cognitive assessment. Both MD and RD correlated negatively with cognition parameters and positively with EDSS. Quantitative DTI parameters not only differentiate between RRMS patients and HCs, but are also associated with disability and mental health of RRMS.
Introduction:
MRI is the most common technique to monitor MS patients, but T2 FLAIR intensities only poorly explain some of the clinical symptoms observed 1,2. Advanced MRI imaging techniques, such as diffusion tensor imaging (DTI) could potentially be used to describe some of the deficits by studying water diffusion in disrupted tracts with parameters like Fractional Anisotropy (FA), Mean Diffusivity (MD), Axial Diffusivity (AD) and Radial Diffusivity (RD) 3,4. DTI allows the evaluation of microstructural integrity of myelin sheath of brain white matter 5. An association can then be established between DTI and clinical symptoms6. This study aims to evaluate and compare DTI parameters in relapsing-remitting MS (RRMS) patients with age and sex-matched healthy controls (HCs), and to correlate these DTI metrics values with clinical symptoms and brain volumetric measures showing the differentiation and significant P-values.Methods:
This observational open-label study involved 37 relapse-onset MS patients aged between 20 to 55 years who had a confirmed diagnosis of RRMS according to the McDonald criteria. Healthy control participants were age and sex-matched to the MS cohort (Table 1). All MRI were undertaken on a 3 Tesla MRI scanner (Prisma, Siemens) located at the Hunter Medical Research Institute, New Lambton Heights, NSW, Australia. A 3D T1 MP-RAGE was used for anatomical reference, and A T2 FLAIR sequence was acquired for assessment of lesion load. Total brain, white matter, grey matter and CSF volumes were calculated by FSL software. The DWI protocol consisted of an echo-planar imaging (EPI) sequence with diffusion weighting b=3000 s/mm2 and 3 diffusion-free (b=0) image volumes. In addition, three b=0 images were acquired with a reversed-phase encoding directions (PA, AP). The DTI pre-processing pipeline 7 was applied to DWI datasets to remove artefacts. DWI datasets were registered to the respective T1 datasets to be able to use segmentations from the T1 image (Figure 1). The DTI tensors were estimated and FA, MD, RD, and AD in total brain white matter (WM) were calculated and analysed.Results:
The correlation and T-test for the DTI metrics and clinical parameters between the two groups are shown in Table 2. There was a statistically significant increase in MD (3.60%), RD (4.50%) and AD (2.70%) and a significant decrease in FA (-4.30%) for total brain-WM in RRMS patients compared to HCs (p<0.01). FA correlated positively with memory (r=0.406) and total-ARCS (r=0.412) in total brain-WM (Table 3). MD correlated negatively with memory (r= -0.372) and positively with EDSS (r=0.368) in total brain-WM. RD correlated negatively with total-ARCS (r= -0.426) and with memory (r= -0.467) while correlated positively with EDSS (r=0.371). Volumetric segmentation indicated a reduction in total volume of the brain (-5%), GM (-4%) and WM (-4%) with a reciprocal increase in CSF by (26%) in RRMS compared with HCs.Discussion:
In this study, we found the DTI metrics are sensitive to microstructural changes in the normal appearing WM. The major findings of this study demonstrate that there is marked reduction of FA values in total brain-WM and significantly increased of MD, AD and RD values in RRMS. Our results are in accordance with previous studies suggesting these microstructural changes may reflect various disease processes in MS patients such as neurodegeneration, axonal loss and gliosis 8,9. It showed a significant increase in MD and RD in total brain-WM of RRMS compared to HCs which are more specific for demyelination and axonal injury. Although we found a reduction in FA values in total brain-WM which is non-specific for demyelination, it can be assumed that the increase in MD and RD, which may represent variable degree of disease activity, could be driven by the low FA values 10,11. Moreover, we found moderate correlation between MD/RD in total brain-WM and EDSS but not with FA which is likely not severely affected in early phase of the disease and has lack of specificity with disease processes 12,13. Cognitive decline is one of the important clinical features of disease progression in MS 14. We found a positive correlation between FA and total ARCS in RRMS compared with HCs. Among other cognitive domains, poor memory and information processing speed are the two common deficits observed in MS patients. We noted a moderate negative correlation between memory and MD/RD in total brain-WM, while FA correlated positively with memory in RRMS. Our results agree with others works highlighting the role of DTI as a viable measure of axonal integrity and cognitive function in MS 15.Conclusion:
There was a statistically significant increase in most of DTI parameters for RRMS patients compared with healthy controls. FA correlated positively with clinical parameters like EDSS and cognitive assessment. Both MD and RD correlated negatively with cognition parameters and positively with EDSS. Quantitative DTI parameters not only differentiate between RRMS patients and HCs, but are also associated with disability and mental health of RRMS.Acknowledgements
This study was supported by an independent grant provided by Biogen and Novartis Australia Pty. Ltd.References
1. Lubetzki, C., et al., Demyelination in multiple sclerosis, in Handbook of clinical neurology. 2014, Elsevier. p. 89-99. 2. Neema, M., et al. (2007),'MRI in multiple sclerosis: what’s inside the toolbox?', Neurotherapeutics. Vol., (4): p. 602-617. 3. Curran, K.M., et al., Quantitative DTI measures, in Diffusion Tensor Imaging. 2016, Springer. p. 65-87. 4. Nucifora, P.G., et al. (2007),'Diffusion-tensor MR imaging and tractography: exploring brain microstructure and connectivity', Radiology. Vol., (2): p. 367-384. 5. Klotz, L., et al. (2019),'Risks and risk management in modern multiple sclerosis immunotherapeutic treatment', Therapeutic Advances in Neurological Disorders. Vol.: p. 1756286419836571. 6. Filippi, M., et al. (2016),'MRI criteria for the diagnosis of multiple sclerosis: MAGNIMS consensus guidelines', Lancet Neurol. Vol., (3): p. 292-303. 7. Veraart, J., et al. (2016),'Denoising of diffusion MRI using random matrix theory', NeuroImage. Vol.: p. 394-406. 8. Hagiwara, A., et al. (2019),'White Matter Abnormalities in Multiple Sclerosis Evaluated by Quantitative Synthetic MRI, Diffusion Tensor Imaging, and Neurite Orientation Dispersion and Density Imaging', American Journal of Neuroradiology. Vol. 9. Guo, A.C., et al. (2002),'Multiple Sclerosis: Diffusion Tensor MR Imaging for Evaluation of Normal-appearing White Matter', Radiology. Vol., (3): p. 729-736. 10. Werring, D.J., et al. (1999),'Diffusion tensor imaging of lesions and normal-appearing white matter in multiple sclerosis', Neurology. Vol., (8): p. 1626-1626. 11. Filippi, M., et al. (2000),'A quantitative study of water diffusion in multiple sclerosis lesions and normal-appearing white matter using echo-planar imaging', Arch Neurol. Vol., (7): p. 1017-21. 12. Sbardella, E., et al. (2013),'DTI Measurements in Multiple Sclerosis: Evaluation of Brain Damage and Clinical Implications', Multiple Sclerosis International. Vol.: p. 11. 13. Tóth, E., et al. (2018),'The contribution of various MRI parameters to clinical and cognitive disability in multiple sclerosis', Frontiers in neurology. Vol.: p. 1172. 14. Ferreira, M.L.B. (2010),'Cognitive deficits in multiple sclerosis: a systematic review', Arquivos de neuro-psiquiatria. Vol., (4): p. 632-641. 15. Meijer, K., et al. (2016),'Patterns of white matter damage are non-random and associated with cognitive function in secondary progressive multiple sclerosis', NeuroImage: Clinical. Vol.: p. 123-131.Figures
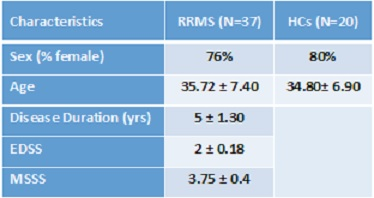
Table 1. Demographic and clinical parameters of study
cohorts of RRMS and HCs groups.
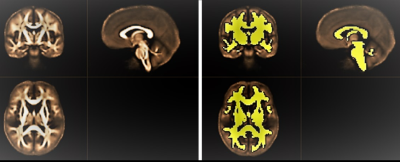
Figure
1. Left: Population-specific FA-template for both groups under study. Right:
white matter mask overlaid on mean FA map.
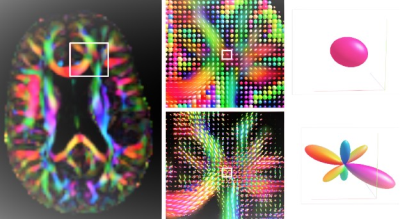
Figure
2. Illustration of fibre orientation distributions (FOD), driving the
registrations in the template creation. Left panel: axial view on the
color-FA map of subject F28. Middle panel: close-up in a region known to
contain multiple crossing fibre bundles. The top image shows diffusion tensors,
the bottom image shows FODs. Right panel: closeup on a single voxel. The
top image shows the diffusion tensor, not capable of distinguishing sub-voxel
structures. Bottom image: the FOD disentangles the diffusion signal into
contributions from multiple fibre bundles.
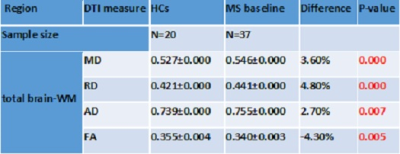
Table
2. Mean values of DTI parameters for the total-brain WM segmentation for RRMS
patients compared to HCs and P-values. MD, RD and AD are in 103 mm2
s, except FA which is unitless.
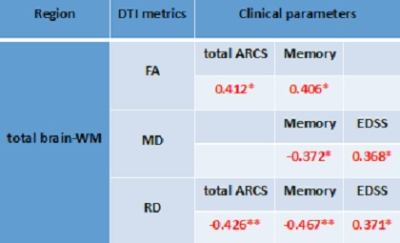
Table
3. Spearman’s correlation between DTI metrics and clinical parameters. Only
statistically significant changes are listed.