4330
Accelerating myelin-water imaging by extracting myelin content from anatomical and diffusion images through machine learning1Department of Radiology and Nuclear Medicine, Maastricht University Medical Center, Maastricht, Netherlands, 2Department of Electrical Engineering, Eindhoven University of Technology, Eindhoven, Netherlands
Synopsis
In this study we aim to accelerate the acquisition time of myelin-water imaging by acquiring fewer slices and applying machine learning to extract myelin-specific information from anatomical (T1w and T2w) and diffusion-weighted imaging (DWI), which are commonly available in many clinical research studies. It is shown that with a 6-fold acceleration (from 7:30min to 1:15min) the myelin content can be reconstructed using neural networks with an agreement to the ground-truth that is comparable to the reproducibility of the scan itself.
Introduction
Myelin is vital for healthy neuronal development, and can therefore provide valuable information regarding neuronal maturation and development as well as insights into disintegration as part of several neurological and neuropsychiatric disorders (1–3). White matter is relatively bright on T1-weighted images due to myelin-bound cholesterol, while the T2-weighted contrast of white matter is relatively low due to motion-restricted protons in the myelin-water (4). Furthermore, though diffusion measures can provide information that are related to the myelin content, it is non-specific and unsuitable for quantification of myelin (5). However, myelin-specific imaging techniques such as myelin-water imaging require relatively long acquisition times (>7 min) and suffer from a low spatial resolution. In this study we aim to accelerate the acquisition time by acquiring fewer slices and applying machine learning to extract myelin-specific information from anatomical (T1w,T2w) and diffusion-weighted imaging (DWI), which are commonly available in many clinical research studies. The obtained sparse myelin-specific information is used as ground truth measurements to train a machine learning algorithm which can subsequently estimate the whole brain myelin-water content.Methods
MRI acquisitionFourteen volunteers (mean age 29y, range 18-39y, 7 males) were scanned on a 3.0 T unit (Philips Achieva, Best, the Netherlands) using a 32-element head coil. T1-weighted fast 3D gradient-echo images were acquired (TR=8.2ms, TE=3.7ms, TI=1010ms, flip angle=8°, voxel size=1mm). Furthermore, DWI was performed (TR=7012ms, TE=74ms, voxel size=2x2x2mm, b-value=1200s/mm2, 66 gradient directions and a single b=0 image). To determine the ground truth myelin content, multi-slice (5 slices acquired simultaneously) GRASE images were acquired for each subject (TR=3000ms, 32 echoes with 10ms echo spacing, range 10-320ms, EPI factor=3, Turbo factor=32, 26 slices acquired in 6 packages, field of view 240x198x130mm, acquisition matrix 160x132, voxel size=1.5x1.5x4mm, SENSE=2, acquisition time 7:30min).
Preprocessing
Myelin-water fraction (MWF) maps were calculated using the regularized non-negative least squares (NNLS) algorithm with a basis set of 120 logarithmically spaced T2 relaxation times between 10 and 2000ms (6). The Extended Phase Graph algorithm and Fourier transform of the slice profile were used to correct for effects of B1 inhomogeneities and imperfect slice profiles (7). The DWI images were first corrected for head displacement, and eddy current induced geometric distortions (ExploreDTI, v4.8.6). Subsequently, the resulting images were registered to the GRASE space and fractional anisotropy (FA) and principal eigenvalues (λ1,λ2,λ3) maps were extracted. The GRASE image with TE=100ms was used as T2w contrast image. T1w images were registered to the TE=10ms GRASE image (SPM12). All images were masked to contain gray and white matter only. Ten white matter regions of interest (ROIs) (major and minor forceps, genu and splenium of the corpus callosum, the whole corpus callosum, and lobular white matter) and 2 gray matter ROIs (cortical gray matter and thalamus) were determined using Freesurfer parcellation and tractography.
Machine learning
For each subject, a neural network was trained using 5 (out of 26) of the simultaneously acquired slices, corresponding to an acceleration factor of 6 (i.e. using only 1 of the 6 acquired packages). The network is trained on a voxel basis, resulting in a large amount of training data for each volunteer (i.e. >100.000). Prior to the training, all input vectors are normalized to the mean of the output (i.e. MWF) vector. Three hidden layers (32 nodes each) with rectified linear activation functions are used resulting in 2,555 trainable parameters. The neural network was trained using 10 epochs. To investigate the added value of DWI in addition to only T1w,T2w images, a neural network with only the T1w,T2w images as inputs was also trained.
Statistical analysis
To assess the agreement between the ground truth and the estimated MWF, the coefficient of variation (CoV, average within-subject standard deviation divided by the overall mean) and intraclass correlation coefficient (ICC, subject variation over the sum of between-subject and within-subject variation) were determined for each ROI. Results are considered to be in good agreement when ICC > 0.80.
Results
Figure 1 shows the ground truth and estimated MWF maps. Figure 2 shows the agreement between ground-truth and estimated MWF of the ten ROIs. Table 1 shows the CoV and ICC of the estimated MWF maps for both neural networks. The MWF map estimated without the DWI information shows a bad agreement with the ground truth (low ICC, high CoV) for several of regions. Especially the splenium benefits from the added DWI information (Figure 2).Discussion & Conclusion
This preliminary study shows the potential of machine learning approaches to extract specific myelin-content from anatomical and DWI scans. The present application could greatly reduce the scanning time of myelin quantification from 7:30min to 1:15min (6-fold), while maintaining an ICC that is comparable to the reproducibility of the full multi-slice GRASE sequence itself (ICC = 0.80) (7). To achieve this, besides T12,T2w images, DWI are required. Furthermore, this study opens up new possibilities, for example, a neural network could be trained to extract myelin content from T1w,T2w and DWI scans directly, without requiring additional myelin-water imaging data. However, the contrasts of T1w,T2w images are qualitative measures and therefore not directly suitable for such an application. Therefore, more research is needed (e.g. intensity normalization procedures) to extract myelin-specific content from anatomical and DWI directly.Acknowledgements
No acknowledgement found.References
1. Drenthen GS, Fonseca Wald ELA, Backes WH, Debeij-van Hall MHJA, Hendriksen J, Aldenkamp AP, Vermeulen RJ, Klinkenberg S, Jansen JFA. Lower myelin-water content of the frontal lobe in childhood absence epilepsy. Epilepsia 2019;60:1689–1696. doi: 10.1111/epi.16280.
2. Kavroulakis E, Simos PG, Kalaitzakis G, Maris TG, Karageorgou D, Zaganas I, Panagiotakis S, Basta M, Vgontzas A, Papadaki E. Myelin content changes in probable Alzheimer’s disease and mild cognitive impairment: Associations with age and severity of neuropsychiatric impairment. J. Magn. Reson. Imaging 2018;47:1359–1372. doi: 10.1002/jmri.25849.
3. Kolind S, Seddigh A, Combes A, et al. Clinical Brain and cord myelin water imaging: a progressive multiple sclerosis biomarker. NeuroImage Clin. 2015;9:574–580. doi: 10.1016/j.nicl.2015.10.002.
4. Glasser MF, Van Essen DC. Mapping Human Cortical Areas In Vivo Based on Myelin Content as Revealed by T1- and T2-Weighted MRI. J. Neurosci. 2011;31:11597–11616. doi: 10.1523/JNEUROSCI.2180-11.2011.
5. Laule C, Vavasour IM, Kolind SH, Li DKB, Traboulsee TL, Moore GRW, MacKay AL. Magnetic resonance imaging of myelin. Neurotherapeutics 2007;4:460–484. doi: 10.1016/j.nurt.2007.05.004.
6. Whittall KP, MacKay AL. Quantitative interpretation of NMR relaxation data. J. Magn. Reson. 1989;84:134–152. doi: 10.1016/0022-2364(89)90011-5.
7. Drenthen GS, Backes WH, Aldenkamp AP, Jansen JFA. Applicability and reproducibility of 2D multi-slice GRASE myelin water fraction with varying acquisition acceleration. Neuroimage 2019;195:333–339. doi: 10.1016/j.neuroimage.2019.04.011.
Figures
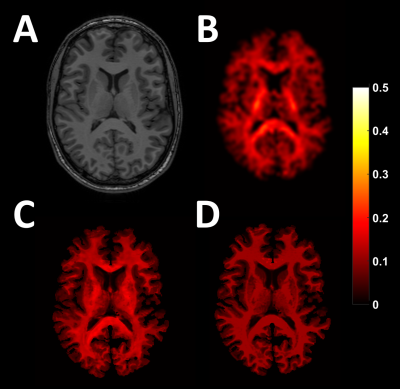
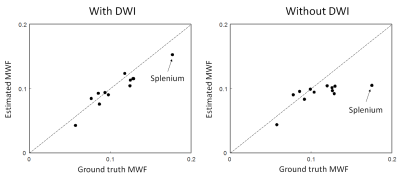
Figure 2: The agreement between the ground-truth and estimated MWF for the ten ROIs is shown for the two cases (with and without DWI). Note that especially the splenium (a region with relative high MWF) benefits from the added DWI information.