4327
Radial diffusion-weighted MRI enables motion-robustness and reproducibility for orthotopic pancreatic cancer in mouse1Department of Radiology, University of Pennsylvania, Philadelphia, PA, United States, 2Pancreatic Cancer Research Center, University of Pennsylvania, Philadelphia, PA, United States, 3Abramson Cancer Center, University of Pennsylvania, Philadelphia, PA, United States
Synopsis
Diffusion weighted (DW)-MRI is sensitive to tumor microenvironment (TME) thus useful for assessing pancreatic cancer responses to stroma-directed drugs, as they change TME by degradation or reduction of extracellular matrix. Motion-sensitive location of pancreatic tumor and fast respiration rate of mice impose a big challenge for quantitative DW-MRI. We compared radial k-space and echo-planar imaging based DW protocol for their accuracy and test-retest reproducibility. EPI-DW consistently underestimates water ADC value at 37C (reference to literature) where radial-DW does not. Better test-retest producibility measured by within-subject CV is obtained with radial-DW compared to EPI-DW.
METHODS: Genetic engineered mouse (GEM) that carries Kras and p53 mutation in pancreas specific Cre allele referred to as KPC model 1 was used. MRI was performed on 4.7T horizontal bore DirectDrive® MR system (Agilent) interfaced with a 12-cm gradient coil. Respiration and core temperature were monitored (SAI Inc, Stonybrook, NY) and the core temperature was maintained at 37±0.2°C by directing warm air into the bore of the magnet. The mouse was placed on top of two 5-mm NMR tubes containing water and butanol, respectively. The test-retest study enrolled KPC mice bearing PDA tumor in the range of 138-929 mm3. After the test study, the mouse was recovered from anesthesia and returned to the cage for 2 hours before re-anesthetized. The RF coil was retuned and recalibrated.
Both Radial and EPI DW-MRI protocol employ a multi-slice 2D spin echo (SE)-DW preparation 2: diffusion sensitizing gradients with a separation time (∆) of 0.0144 sec applied for a duration (δ) of 0.009 sec to +x, +y, +z and then -x, -y, -z with varied amplitude, resulting in five b-values (0.64, 535, 1071, 1478, 2141 s/mm2); FOV=32 x 32 mm2. Radial-DW acquisition includes 256 views (spokes), each having 128 points; one signal average. For EPI-DW, the entire k-space (128 phase encoding lines) was sampled in 4 shots, each acquiring 32 echoes per TR of 250 msec; 16 signal averages were used to obtain similar SNR as Radial-DW images. Abdominal images of mice acquired by EPI-DW protocol with 1- or 2-shot have unacceptable SNR. Respiration gated and non-gated acquisition were compared in both protocols. Test-retest statistics including within-subject coefficient-of-variation (CVWS) are computed 3,4.
RESULTS: Due to motion susceptible location of pancreatic tumor, respiration-gating implemented with SE-DW protocol does not adequately suppress respiratory motion artefacts (Fig 1A) therefore additional motion resistant mechanism is necessary for murine abdominal DW-MRI. Both Radial-DW and EPI-DW protocol (with respiration gating) lead to motion-free diffusion-weighted images even at very high b-values (Fig 1B-1C). Comparing to Radial-DW, EPI-DW results in ghosting artefacts around the butanol phantom (white arrows in Fig 1C). The ADC of water phantom measured by radial-DW is almost identical to literature value of 3.2 x 10-3 mm2/s at 37°C 5.
Bland-Altman plots, where ADC values of water phantom measured by Radial or EPI-DW protocol were compared with the literature value are shown in Fig 2: in plot derived from Radial-DW (2A), bias is minimal and ±95% CI (confidence interval) lines are evenly distributed around x-axis; In contrast, in plot from EPI-DW (2B), equality line is outside the ±95% CI limit, suggesting a systemic error introduced by EPI-DW. Since all data points are distributed on the left of the literature value (dotted line), EPI-DW consistently underestimates water ADC.
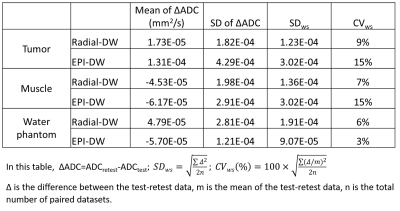
Statistics for test-retest studies (Table-1) show that CVWS of tumor and muscle ADC <10% achieved by Radial-DWI, suggesting an excellent test-retest reproducibility for a motion susceptible tumor compared to CVWS of 15% by EPI-DW. ADC values of pancreatic tumor measured by Radial- and EPI-DW are highly correlated (r2 =0.82, Fig 3A). Bland-Altman plot of test-retest ADC values revealed that Radial-DW leads to a smaller bias and better agreement (smaller width between ±95% CI) than ADC from EPI-DW protocol (Fig 3B-3C).
DISCUSSION: Both Radial- and EPI-DW protocol (in combination with respiration gating) can effectively remove motion artefacts from DW images although ghosting persists in EPI-DW images likely due to multi-shot k-space sampling. A systemic error in ADC of water (reference to literature) is only observed with EPI-DW but not with Radial-DW protocol. Furthermore, Radial-DW leads to better test-retest producibility with lower CVWS compared to EPI-DW.
CONCLUSION: Our data revealed that motion-robustness for DW-MRI of orthotopic pancreatic tumor in mice can be obtained by Radial-DW protocol with high degree of accuracy and good test-retest reproducibility. Out study is consistent with recent QIBA recommendations for improved precision of DW-MRI biomarkers for oncology trial 3.
Acknowledgements
This study was partially supported by U24CA231858 (Penn Quantitative Imaging Resource for Pancreatic Cancer), R21CA198563 and R01CA211337.References
1. Hingorani SR, Petricoin EF, Maitra A, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell 2003;4(6):437-450.
2. Liu T, Choi H, Zhou R, Chen IW. RES blockade: A strategy for boosting efficiency of nanoparticle drug. Nano Today 2015;10(1):11-21.
3. Shukla-Dave A, Obuchowski NA, Chenevert TL, et al. Quantitative imaging biomarkers alliance (QIBA) recommendations for improved precision of DWI and DCE-MRI derived biomarkers in multicenter oncology trials. J Magn Reson Imaging 2019;49(7):e101-e121.
4. Ge X, Quirk JD, Engelbach JA, et al. Test-Retest Performance of a 1-Hour Multiparametric MR Image Acquisition Pipeline With Orthotopic Triple-Negative Breast Cancer Patient-Derived Tumor Xenografts. Tomography : a journal for imaging research 2019;5(3):320-331.
5. Patlak CS, Hospod FE, Trowbridge SD, Newman GC. Diffusion of radiotracers in normal and ischemic brain slices. J Cereb Blood Flow Metab 1998;18(7):776-802.
Figures
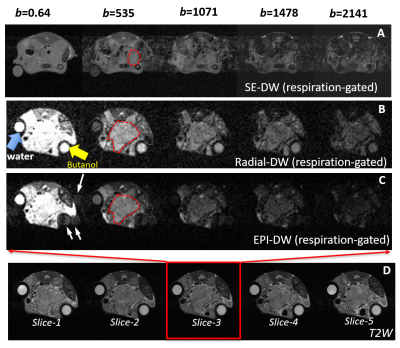
Figure 1 Comparison of Radial- vs. EPI-DW for motion suppression and ghosting.
DW-MR images obtained from respiration-gated SE- (A), Radial- (B) and EPI-DW (C) protocol using indicated b-values. White arrows in A point to motion artifacts at b=500 and above and to ghosting artefacts in EPI-DW images (C). Red frame and arrows in D indicate that of the five T2W images, slide-3 corresponds to DW-images displayed in B and C with different b-values. DW-images in A are from a different mouse.
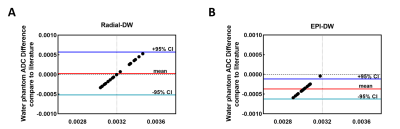
Figure 2 Accuracy of ADC value of water phantom measured by Radial- vs. EPI-DW Protocol in comparison with literature
Bland-Altman plot of ADC values from Radial-DW (A) or EPI-DW (B) in comparison with literature value (3.2 x 10-3 mm2/s) at 37°C.

Figure 3 Correlation and test-retest ADC values in tumor and muscle in comparison of radial vs. EPI DW-MRI Protocol
The tumor ADC values measured from Radial- and EPI-DW are highly correlated (A) (r2=0.82). Bland-Altman plots of tumor and muscle ADC value between test and retest data were shown for Radial-DW (B) and EPI-DW (C) protocol. More details in Table 1.