4325
Feasibility and evaluation of whole brain single-slab 3D DWI and comparison to 2D multi-slice DWI1National Institutes of Health, Bethesda, MD, United States
Synopsis
While most brain imaging sequences now favor their 3D counterparts, diffusion imaging is an exception. This is due to large diffusion gradients resulting in increased sensitivity to motion exhibited by 3D acquisition. Prior schemes have used limited brain coverage and/or triggering or acquired multiple 3D slabs along with modified reconstruction schemes. The modified sequence used here employs first-order motion compensated diffusion gradients in addition to real-time alignment to acquire whole brain 3D-DWI images as a single slab. Relatively shorter TE (using enhanced gradients) and TR along with other modifications result in faster, reduced artifact diffusion images while providing higher SNR.
Introduction
MRI sequences have been trending in favor of 3D acquisition over 2D multi-slice (MS) imaging. A majority of brain imaging sequences (including Flair, T1 and T2-w, SWI, MPRAGE) now preferably employ their 3D counterparts. Advantages to 3D acquisition (relative to 2D) include finer slice definition, higher SNR/time and possibility of obtaining isotropic image data sets for processing.Diffusion imaging has been an exception to this trend, in large part due to the difficulty of obtaining artifact-free images. These artifacts arise because of sensitivity of 3D acquisitions to physiologic brain motion and CSF pulsations. In the 3D case, motion related phase inconsistencies induced by diffusion gradients aggregate along the slice direction, a problem not present in the 2D case. Previous work in 3D has aimed at high resolution limited coverage of specific regions of the brain and using longer scan times1,2. Other works have employed multiple 3D slabs (relying on through plane component of motion-induced phase to change insignificantly between TRs) and modified reconstruction to stitch together whole brain diffusion images3-5. A majority of these implementations required extensive reconstruction based post-processing, difficult in a clinical environment. In this work, we implemented a clinically feasible whole brain 3D diffusion weighted imaging scheme and compared it to 2D multi-slice DWI.
Methods
Several sequence design modifications were utilized for 3D-DWI acquisition. Modifications related to reducing motion were as follows:- Intra-TR motion was reduced through use of first-order motion compensated diffusion encoding gradients using enhanced gradient strength (max: 80 mT/m, SR100) to keep TE relatively short. In addition, shorter TR was employed.
- Inter-TR motion related phase effect was reduced by monitoring motion on the fly by registering to a previous volume and adjusting excitation and acquisition through phase offsets and rotation matrix.
Scanning:
Seven volunteers (3M, 4F) were scanned under an IRB approved protocol on a Philips 3T scanner (equipped with dual mode gradients) using a 32-channel head coil.
Scan parameters were as follows:
MS-DWI: FOV=23x23cm2, res:2x2x2mm3, slice gap=0, TR/TE=8190/75ms, ss-EPI, HFF=0.68, b=1000s/mm2, SENSE(y)=2, ~74 slices, scan time:1:42.
3D-DWI: FOV=23x23cm2, res:2x2x2mm3, TR/TE=325/78 ms, NSA=2, ss-EPI, HFF=0.64, b=1000s/mm2, SENSE(y)=2.5, SENSE(z)=2, ~74 slices, scan time:3:15.
Processing:
To compare MS and 3D-DWI, post-processing was performed in SPM and Matlab®. The following steps were carried out.
- All images were registered to a common frame.
- The b=0s/mm2 images were segmented to provide GM and WM maps.
- These maps were employed on directional images to obtain segmented maps.
- Directional images were scaled by b=0 image values on a pixel by pixel basis.
- Values were compared between MS-DWI and 3D.
- Directional images were combined to produce trace and ADC maps.
- Relative SNR measurements were done for b=0 images by drawing corresponding ROIs in sub-cortical regions and outside the brain for several matching slices.
Results
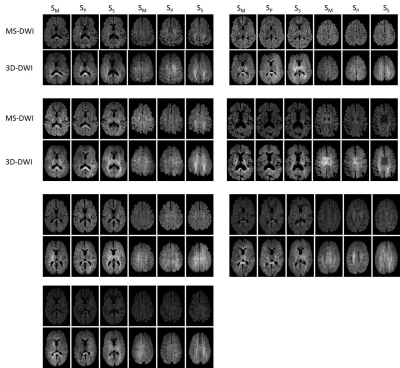
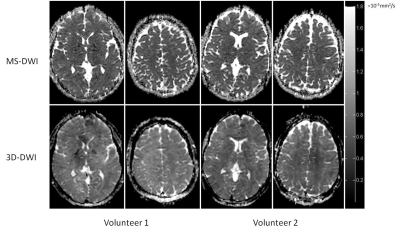
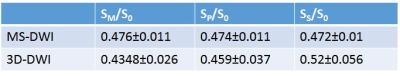
Table 1 shows values measured in GM+WM for scaled directional images across all volunteers. Global difference between MS-DWI directional SM (measurement axis), SP (phase) and SS (slice-encoding) images and corresponding 3D-DWI images was 8.7%, 3.2% and -10.2%, respectively. Table 2 provides (mean+std) global ADC values for all volunteers. Difference between MS-DWI and 3D-DWI gray matter ADC was 12.6% and for white matter ADC was -4.6%. SNR corrected for scan time was 1.7 times higher for 3D-DWI compared to MS-DWI.Sagittal images were reformatted in the transverse plane. Figure 1 shows two such reformatted slices of diffusion weighted images along M, P and S directions for seven volunteers for the two acquisition schemes (MS and 3D). Figure 2 shows trace $$$\sqrt[3]{S_M\cdot S_P\cdot S_S}$$$ images for two volunteers. Figure 3 shows sample ADC maps for two volunteers.
Discussion
In this work, use of gating or multi-slab acquisitions was eschewed in favor of speed (since increased scan times defeat the very purpose of curtailing motion artifacts) and motion-compensated gradients along with real-time volume alignment. Nevertheless, large phase inconsistencies along z direction as a result of residual eddy currents, motion and table vibration can result in ghosting or signal loss. Reduction in echo time through use of enhanced gradients can be prone to increased eddy currents and mechanical vibration related artifacts. Through plane pulsatile CSF motion can corrupt neighboring slices due to phase encoding along the z direction. This can affect quantitative diffusion imaging.Quantitatively, diffusion images along the slice encode direction showed the highest bias when compared with their MS counterpart. This bias on our system was a result of employing enhanced gradients which was confirmed by scanning a phantom at two different gradient strengths. For the sequences used here, SAR for 3D-DWI was lower at ~25% of maximum when compared to MS-DWI (~71%). PNS was 65% for 3D-DWI and 79% for MS-DWI.
In summary, full brain single-slab 3D-DWI with relatively high resolution, and good image quality in a clinically feasible scan time, was achieved. Improved quantitative accuracy will be possible with future improvements in hardware and mechanical stability. Extension of 3D-DWI to DTI currently poses challenges related to increased scan time and mechanical stability.
Acknowledgements
No acknowledgement found.References
1. Golay X, Jiang H, van Zijl PC, Mori S. High-resolution isotropic 3D diffusion tensor imaging of the human brain. Magn Reson Med 2002;47(5):837-843.
2. Frank LR, Jung Y, Inati S, Tyszka JM, Wong EC. High efficiency, low distortion 3D diffusion tensor imaging with variable density spiral fast spin echoes (3D DW VDS RARE). Neuroimage 2010;49(2):1510-1523.
3. Engstrom M, Skare S. Diffusion-weighted 3D multislab echo planar imaging for high signal-to-noise ratio efficiency and isotropic image resolution. Magn Reson Med 2013;70(6):1507-1514.
4. Chang HC, Sundman M, Petit L, et al. Human brain diffusion tensor imaging at submillimeter isotropic resolution on a 3Tesla clinical MRI scanner. Neuroimage 2015;118:667-675.
5. Wu W, Poser BA, Douaud G, et al. High-resolution diffusion MRI at 7T using a three-dimensional multi-slab acquisition. Neuroimage 2016;143:1-14.
Figures