4323
Q-Space Trajectory Imaging Using the MAGNUS High-Performance Head Gradient1Electrical Engineering, Stanford University, Stanford, CA, United States, 2Radiology, Stanford University, Stanford, CA, United States, 3Hospital for Special Surgery in Manhattan, New York, NY, United States, 4GE Global Research, Niskayuna, NY, United States
Synopsis
In this work, q-space trajectory imaging was implemented on a whole-body system equipped with a high-performance head-only gradient system capable of 200 mT/m maximum gradient amplitude and 500 T/m/s slew rate. The improved gradient performance enabled the acquisition of q-space trajectory imaging with sub-millimeter in-plane resolution and reduced voxel volume compared to previously published work, while simultaneously improving head coverage, and reducing susceptibility induced image distortions.
Introduction
Increased gradient amplitude, slew rate and duty cycle achievable using high-performance gradient systems are especially beneficial for advanced diffusion encoding methods, which expand the microstructural information captured by the diffusion experiment. Q-space trajectory imaging1 encodes diffusion along multiple directions before the image readout to separate the effects of orientation dispersion and microscopic diffusion anisotropy. Although microscopic diffusion anisotropy offers a new type of contrast that may improve sensitivity to changes in brain tissue microstructure, encoding along multiple gradient directions requires extended diffusion encoding time, resulting in long echo times and limiting the achievable spatial resolution to 2-3mm isotropic for in vivo human imaging on clinical whole-body MRI scanners2,3. In addition, the slice coverage is often poor due to increased strain on the gradient power supply and heat dissipation caused by the diffusion gradient waveforms. In this work, we demonstrate the improved spatial resolution of q-space trajectory imaging enabled by a high-performance head-only gradient insert coil with higher PNS thresholds, allowing the use of higher slew rates and amplitudes compared to clinical whole-body systems.Methods
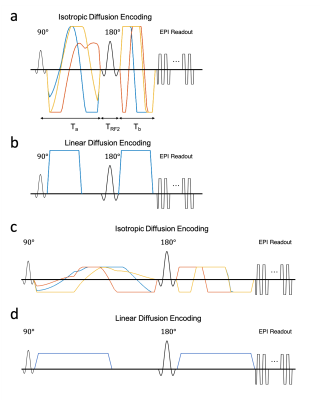
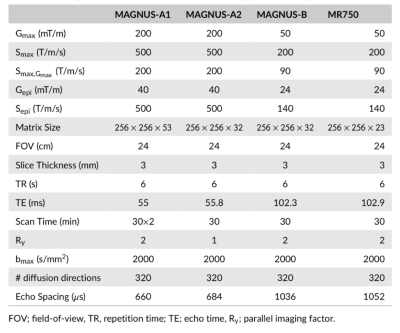
Waveform Design:Isotropic diffusion encoding4 waveforms were designed using the numerical optimization of gradient waveforms (NOW) toolbox with second order concomitant field correction5,6 as shown in Figure 1a,c. The designs minimized the echo-time to achieve isotropic diffusion weighting with a b-value of 2500s/mm2 at an imaging resolution of 0.9×0.9×3mm for both a high-performance head-only gradient coil7,8(MAGNUS, GE Global Research: Gmax=200mT/m, Smax=500T/m/s) and a standard gradient coil (MR750, GE Healthcare: Gmax=50mT/m, Smax=200T/m/s).
Data Acquisition:
Four scans were conducted on a single subject with IRB approval to compare QTI acquisitions at different gradient performance levels. The diffusion acquisition scheme consisted of 30, 30 and 60 linear encodings (Fig. 1b,d) at 500, 1500, and 2000 s/mm2 and 8, 16, 36, and 90 isotropic diffusion encodings at 500, 1500, 1800 and 2000s/mm2. An additional 22 non-diffusion weighted volumes were evenly spaced throughout the acquisition for a total of 321 image volumes. The first scan was conducted on the MAGNUS system with full gradient capabilities using the diffusion waveforms shown in Figure 1a,c and acquisition parameters shown in Table 1: MAGNUS-A1. The second scan used identical diffusion parameters, but without in-plane parallel imaging (Table 1: MAGNUS-A2) to match the effective echo spacing of the gradient-limited acquisitions. The third scan was conducted on the MAGNUS system with gradients derated to approximate the performance of the conventional gradient coil using the diffusion waveforms shown in Figure 1b,d and acquisition parameters in Table 1: MAGNUS-B. The final acquisition was conducted on the MR750 system using the diffusion waveforms in Figure 1b,d and acquisition parameters in Table 1: MR750. T1-weighted MPRAGE images were also acquired.
Data Analysis:
Diffusion weighted images were corrected for eddy current distortion, bulk motion and susceptibility distortions. The orientation averaged diffusion signal was fitted to the DIVIDE model using a custom gradient descent algorithm in MATLAB (Mathworks) to compute the microscopic fractional anisotropy. For each subject MPRAGE images were segmented using FreeSurfer to generate regions of interest in the white matter, corpus callosum, cortex, thalamus, putamen and caudate. Images were aligned to MPRAGE space using boundary-based registration “epi_reg” from FSL with 12 degrees of freedom. Temporal SNR maps were calculated using the repeated B0 and isotropic diffusion weighted images.
Results
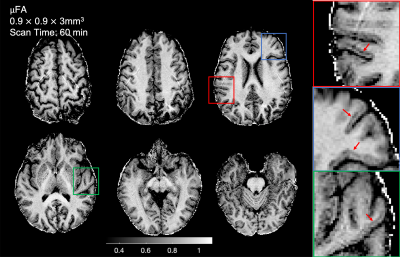
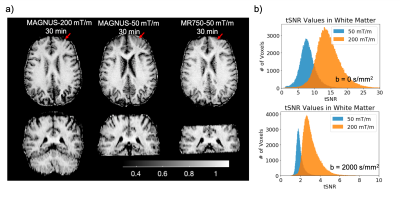
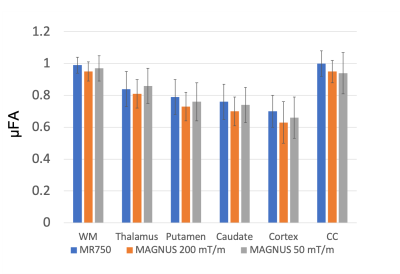
Figure 2 shows representative slices of microscopic fractional anisotropy maps generated from the MAGNUS-A1 acquisition with 0.9×0.9mm2 in-plane resolution. The improved gradient performance resulted in improvements in SNR, increased slice coverage, and reduced image distortions as shown in Figure 3a. Comparing acquisitions with the same readout time (Fig. 3b) yielded an average 1.6-1.8× improvement in SNR within the white matter for the high performance system(MAGNUS-A2) compared to the gradient limited acquisitions (MAGNUS-B and MR750). Figure 4 compares the average µFA measurements within regions of interest in the white matter, thalamus, putamen, caudate, cortex and corpus callosum between the QTI acquisitions. The relative µFA values followed the same trend on all three acquisitions. However, the measurements made using the MAGNUS 200mT/m acquisition were slightly lower than measurements from the MAGNUS 50mT/m and MR750 acquisition with the exception of the corpus callosum.Discussion
Stronger gradients, higher slew rates and higher duty cycles afforded by head-only gradient systems greatly improves SNR efficiency of QTI acquisitions enabling imaging at finer spatial resolutions. The 2.43mm3 voxel volume is substantially reduced compared to previous µFA maps (8-27mm3), and resolves fine structures in the brain including details in the white matter/gray matter boundary. The SNR improvement measured was close to the expected SNR gain (1.87×) due to the reduced echo time(102.3 to 55.8ms) assuming a T2 in white matter of 70ms. The slight discrepancy between the µFA values from the 200mT/m acquisition compared to the 50mT/m acquisitions could be due to an increase of µFA measures with echo-time9, time-dependent diffusion effects due to shorter effective diffusion times of the 200mT/m waveform, and/or a potential positive bias in the 50mT/m measurements caused by low SNR.Conclusion
The improved gradient performance enabled the acquisition of q-space trajectory imaging with sub-millimeter in-plane resolution and reduced voxel volume compared to previously published work, while simultaneously improving head coverage, and reducing susceptibility induced image distortions.Acknowledgements
Funding provided by an NSF-GRFP, CDMRP W81XWH-16-2-0054, NIH: R01 NS095985, R01 MH111444, R21 MH116484, S10 RR026351, P41 EB015891, GE Healthcare and the Dana Foundation.References
1. Westin CF, Knutsson H, Pasternak O, Szczepankiewicz F, Ozarslan E, van Westen D, Mattisson C, Bogren M, O'Donnell LJ, Kubicki M and others. Q-space trajectory imaging for multidimensional diffusion MRI of the human brain. Neuroimage 2016;135:345-62.
2. Yang G, Tian Q, Leuze C, Wintermark M, McNab JA. Double diffusion encoding MRI for the clinic. Magn Reson Med 2018;80(2):507-520.
3. Szczepankiewicz F, Lasic S, van Westen D, Sundgren PC, Englund E, Westin CF, Stahlberg F, Latt J, Topgaard D, Nilsson M. Quantification of microscopic diffusion anisotropy disentangles effects of orientation dispersion from microstructure: applications in healthy volunteers and in brain tumors. Neuroimage 2015;104:241-52.
4. Mori S, van Zijl PC. Diffusion weighting by the trace of the diffusion tensor within a single scan. Magn Reson Med 1995;33(1):41-52.
5. Baron CA, Lebel RM, Wilman AH, Beaulieu C. The effect of concomitant gradient fields on diffusion tensor imaging. Magn Reson Med 2012;68(4):1190-201.
6. Szczepankiewicz F, Nilsson M. Maxwell-compensated waveform design for asymmetric diffusion encoding. 2018; Paris, France. Intl Soc. for Magn Reson in Med.
7. Tan ET, Hua Y, Fiveland EW, Vermilyea ME, Piel JE, Park KJ, Ho VB, Foo TKF. Peripheral nerve stimulation limits of a high amplitude and slew rate magnetic field gradient coil for neuroimaging. Magnetic Resonance in Medicine 2020;83(1):352-366.
8. Foo TKF TE, Vermilyea M et al. Highly efficient head-only magnetic field insert gradient coil for achieving simultaneous high gradient amplitude and slew rate at 3.0T (MAGNUS) for brain microstructure imaging. Magnetic Resonance in Medicine 2019.
9. Yang GK M, JA. Echo Time Dependence of Double Diffusion Encoding Measurements of Microscopic Diffusion Anistropy. 2018 2018; Paris, France. International Society for Magnetic Resonance in Medicine.
Figures