4316
Oscillating Gradient (OG) Prepared 3D-GRASE Sequence for Improved OG-Diffusion MRI1Key Laboratory for Biomedical Engineering of Ministry of Education, Department of Biomedical Engineering, College of Biomedical Engineering & Instrument Science, Zhejiang University, Hangzhou, China, 2Johns Hopkins University School of Medicine, BALTIMORE, MD, United States, 3MR Collaboration, Siemens Healthcare Ltd., Shanghai, China
Synopsis
Oscillating gradient enables access of short diffusion times for time-dependent diffusion MRI (dMRI), but poses challenges for clinical use, including limited oscillating frequencies and b-values, low SNR, and relatively long scan times. This study proposes a 3D oscillating gradient prepared gradient spin-echo sequence (OGprep-GRASE) to improve the SNR and shorten the acquisition time for OG-dMRI. The proposed sequence reduced the scan time by a factor of 1.38 and increased the SNR by 1.74 times, compared with the existing 2D echo-planar imaging (EPI) approach, leading to improved diffusion tensor reconstruction. Diffusivity measurements showed similar time-dependency using the GRASE and EPI sequences.
Introduction
Oscillating gradient diffusion MRI (OG-dMRI) is an essential approach to achieve short diffusion time for time-dependent dMRI 1,2. However, this technique faces major challenges for clinical applications due to the need for strong gradients 3,4. Despite the limited oscillating frequencies and b-values accessible with clinical gradients, recent studies indicated that accurate microstructural modeling was still possible on clinical systems 5. However, additional issues impeding the clinical translation of OG-dMRI remain: 1) long echo-times (TEs) and low signal-to-noise ratios (SNRs) due to the use of long oscillating gradients with multiple cycles to reach a certain b-value; and 2) long scan times due to the long repetition times (TRs) needed to reduce the duty cycle of repetitively applied strong gradients in 2D multislice acquisition. In this study, we designed a 3D oscillating gradient prepared gradient spin-echo sequence (OGprep-GRASE) to achieve faster OG-dMRI acquisition with a higher SNR on a clinical 3T system.Methods
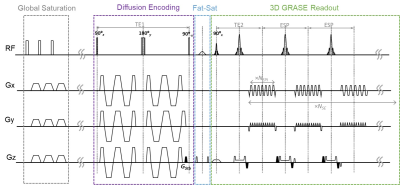
Pulse sequence: Figure 1 summarizes the 3D OGprep-GRASE sequence. In the diffusion preparation module, a pair of trapezoid-cosine oscillating gradients (or pulsed gradients) was embedded in the 90ºx- 180ºy- 90º-x chain. Stabilizer gradients were used before the tip-up 90° pulse and around each EPI readout to mitigate the phase-error-dependent signal modulation 6,7. The 3D GRASE readout was achieved by echo-planar imaging (EPI) encoding in the Y direction (NEPI) and turbo spin-echo encoding in the Z direction (NSE). Segmented readout was performed in the EPI direction.Data acquisition and analysis: Seven healthy young male volunteers (20-25 years old) were enrolled. Two sets of experiments were performed on a 3T MAGNETOM Prisma scanner (Siemens Healthcare, Erlangen, Germany) with a maximum gradient of 80mT/m.
1) To compare the scan times and SNRs of the 3D OGprep-GRASE and 2D OG-EPI sequences, OG-dMRI was performed at an oscillating frequency of 50Hz, resolution of 2.75×2.75×3mm3, FOV=220×220mm, b=500 s/mm2, 12 directions, 2 repetitions, with the following schemes: a) single-shot GRASE (NEPI=80, NSE=10, TE1/TE2/ESP/TR=124/33.6/33.6/3000 ms) and EPI with 10 slices (TE/TR=158/4200ms); and b) 2-shot GRASE (NEPI=80, NSE=20, segmented in EPI direction, TE1/TE2/ESP/TR=124/22.9/22.9/3000 ms) and EPI with 20 slices (TE/TR=147/8400ms). A 30-slice protocol was acquired in some of the subjects. Multi-shot GRASE data were reconstructed with multiplexed sensitivity-encoding (MUSE) for phase-error correction 8. The SNR was calculated by the standard deviation of the subtraction image of two b0 images normalized to the mean of the b0 images, and overall image quality was evaluated visually based on reconstructed diffusion tensor metrics.
2) To test the reliability of time-dependent diffusivity measurements, single-shot OGprep-3D GRASE was performed with an oscillating frequency of 25Hz (1 cycle) and 50Hz (2 cycles) and pulsed gradient (PG) prepared-3D GRASE with δ=20ms and Δ=30 and 60ms, b=600 s/mm2, 6 directions, TE1/TE2/TR=84/32/3000 ms, resolution = 2.75×2.75×5mm3, to compare with the 2D OG-EPI sequence using matched parameters. The time-dependency and sequence difference was assessed by two-way analysis of variance (ANOVA).
Results
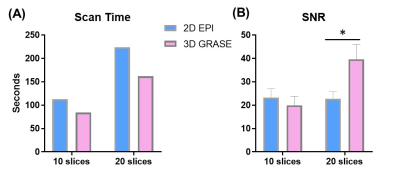
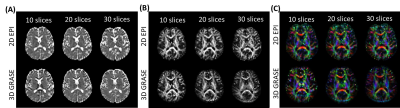
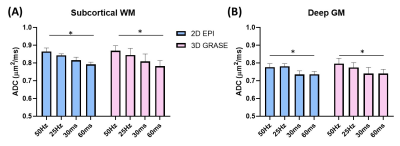
OGprep-3D GRASE accelerated the OG-dMRI acquisition by a factor of 1.34 and 1.38 times for the 10-slice and 20-slice acquisition protocols, compared with the 2D EPI sequence (Figure 2A). For the 10-slice protocol, the GRASE and EPI sequences showed similar SNRs; the SNR was doubled for 3D GRASE as the imaging volume increased to 20 slices while the SNR of 2D EPI data remained the same (Figure 2B). The enhanced SNR led to improved DTI reconstruction, which can be visualized from the apparent diffusion coefficient (ADC) maps, fraction anisotropy (FA) maps, and direction-encoded colormaps (DEC) (Figure 3). ADCs of the OG-dMRI (50Hz and 25Hz) and PG-dMRI (30ms and 60ms) data were obtained in the subcortical white matter and the deep gray matter, and these were compared between the 3D OGprep-GRASE and 2D EPI sequences. ADC values from both sequences demonstrated significant time-dependency in the white and gray matter regions (p<0.0001), and no significant sequence differences were found by two-way ANOVA (Figure 4).Discussion and Conclusion
An OGprep-GRASE sequence was designed for time-dependent dMRI studies on a clinical system. This design provides advantages over conventional 2D multislice sequences. First, in 2D EPI-based dMRI, the diffusion gradients are repetitively applied for each slice, leading to a high duty cycle and long TR for OG-dMRI, especially when the slice coverage is large; whereas in 3D GRASE, the diffusion gradient is only applied once for the entire volume. Second, the use of 3D acquisition improves the SNR compared to the 2D multislice approach. These advantages in scan time and SNR were supported by our experimental results.A diffusion-prepared strategy is used to separate the diffusion encoding, which is important for OG-dMRI. Otherwise, the echo spacing between the refocusing pulses (Figure 1) has to be long, in order to match TE1 that accommodates the long oscillating gradients. The prepared strategy reduced the SNR by half due to the use of stabilizers, but the SNR benefits remained substantial as the imaging volume increased. The phase errors between shots was corrected by MUSE alone, limiting the number of shots to 2-3. Further work includes adding navigators 9 to assist phase correction, which may enable high-resolution 3D dMRI with more shots. Parallel imaging and other fast imaging techniques may also be incorporated to accelerate the 3D encoding.
Acknowledgements
This work was supported by the Natural Science Foundation of China (61801424, 81971606, and 91859201) and the Ministry of Science and Technology of the People’s Republic of China (2018YFE0114600).References
1. Schachter M, Does MD, Anderson AW, Gore JC. Measurements of restricted diffusion using an oscillating gradient spin-echo sequence. Journal of magnetic resonance 2000;147(2):232-237.
2. Gore JC, Xu JZ, Colvin DC, Yankeelov TE, Parsons EC, Does MD. Characterization of tissue structure at varying length scales using temporal diffusion spectroscopy. NMR in biomedicine 2010;23(7):745-756.
3. Van AT, Holdsworth SJ, Bammer R. In vivo investigation of restricted diffusion in the human brain with optimized oscillating diffusion gradient encoding. Magn Reson Med 2014;71(1):83-94.
4. Baron CA, Kate M, Gioia L, Butcher K, Emery D, Budde M, Beaulieu C. Reduction of Diffusion-Weighted Imaging Contrast of Acute Ischemic Stroke at Short Diffusion Times. Stroke 2015;46(8):2136-2141.
5. Xu J., Jiang X., Li H., Arlinghaus LR, McKinley E.T., Devan SP, Hardy B.M., Xie J., Kang H., Chakravarthy A.B., J.C. G. Magnetic resonance imaging of mean cell size in human breast tumors. https://arxivorg/abs/190507818 2019.
6. Alsop DC. Phase insensitive preparation of single-shot RARE: application to diffusion imaging in humans. Magn Reson Med 1997;38(4):527-533.
7. Van AT, Cervantes B, Kooijman H, Karampinos DC. Analysis of phase error effects in multishot diffusion-prepared turbo spin echo imaging. Quant Imaging Med Su 2017;7(2):238-250.
8. Chen NK, Guidon A, Chang HC, Song AW. A robust multi-shot scan strategy for high-resolution diffusion weighted MRI enabled by multiplexed sensitivity-encoding (MUSE). NeuroImage 2013;72:41-47.
9. Mori S, van Zijl PC. A motion correction scheme by twin-echo navigation for diffusion-weighted magnetic resonance imaging with multiple RF echo acquisition. Magn Reson Med 1998;40(4):511-516.
Figures