4315
Intravoxel incoherent motion analysis of the brain with second-order motion-compensated diffusion encoding1Faculty of Health Sciences, Institute of Medical, Pharmaceutical and Health Sciences, Kanazawa University, Kanazawa, Japan, 2Philips Japan, Tokyo, Japan, 3Radiology Division, Kanazawa University Hospital, Kanazawa, Japan, 4Department of Radiology, Kanazawa University Graduate School of Medical Sciences, Kanazawa, Japan
Synopsis
In this study, we compared diffusion parameters with intravoxel incoherent motion (IVIM) analysis of the brain between second-order motion-compensated (2nd-MC) and conventional (non-MC) diffusion encoding schemes. Perfusion-related diffusion coefficient with non-MC was strongly affected by bulk motion in the pons which has the largest motion in the brain. By contrast, the 2nd-MC diffusion gradients compensated the bulk motion-induced signal loss and improved the fitting accuracy of biexponential model. The 2nd-MC diffusion encoding reduces the bulk motion effect on IVIM analysis of the brain, thereby improving the measurement accuracy.
Introduction
Intravoxel incoherent motion (IVIM) analysis of the brain has shown to have clinical relevance for the diagnosis of neurological disorders.1 However, some issues still need to be addressed to make the diffusion parameters more reliable. Especially, physiological variability due to brain pulsation (i.e., bulk motion) as well as pulsatile blood flow during diffusion sensitization leads to artefactual phase dispersion and thus overestimation of the apparent diffusion coefficient.2 Recently, a number of groups have reported that second-order motion-compensated (2nd-MC) diffusion encoding successfully reduced the sensitivity to bulk motion and yielded robust estimation of diffusion parameters in cardiac diffusion-weighted imaging.3,4 We hypothesized that measurement accuracy of IVIM parameters in the brain can be improved with 2nd-MC diffusion encoding. Therefore, to obtain more accurate diffusion information, we performed IVIM analysis of the brain with the 2nd-MC diffusion gradients and compared the diffusion parameters between the 2nd-MC and conventional diffusion encoding schemes.Methods
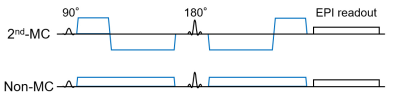
On a 3.0-T MRI, eight healthy volunteers (6 males and 2 females; mean age, 24.8 years; range, 22-34 years) were scanned. Single-shot diffusion echo-planar imaging of the brain was performed with the 2nd-MC and conventional (non-MC) diffusion gradients (Figure 1). Transverse diffusion-weighted images were obtained with the following imaging parameters: repetition time, 4600 ms; echo time, 143 ms; slice thickness, 6 mm; field of view, 256 mm; imaging matrix, 128 × 128; number of signals averaged, 2; sensitivity encoding factor, 2.1; half-scan factor, 0.7; and b-values, 0, 10, 20, 30, 50, 100, 200, 400, 600, 800, and 1000 s/mm2.Regions of interests (ROIs) were manually drawn in the pons, caudate nucleus, and frontal white matter. Then, mean signal intensities in the ROIs were fitted with the following biexponential function to calculate perfusion-related diffusion coefficient (D*), the fraction (F), and restricted diffusion coefficient (D):
$$$ S = S_{0}\cdot[F\cdot\exp(-bD^{*}) + (1-F)\cdot\exp(-bD)]$$$,
where S and S0 are signal intensities at a given b-value and without diffusion encoding, respectively. We used a two-step approach to improve the accuracy and robustness of the analysis: D was initially determined using monoexponential function in b-values over 200 s/mm2. Then, with the D fixed, D* and F were derived using all b-values. The fitting procedure was performed at MATLAB using the Levenberg-Marquardt nonlinear least-squares algorithm.
Normalized root-mean-square error (nRMSE) was calculated to evaluate the goodness-of-fit of biexponential model for the measured data from each diffusion encoding scheme5:
$$$ nRMSE = \frac{\sqrt{RSS/n}}{S_{0} - S_{1000}}$$$,
where RSS is the residual sum of squares and n is the number of measured data. S0 and S1000 are signal intensities at b-values of 0 and 1000 s/mm2, respectively. A smaller nRMSE indicates better fitting quality.
We compared these diffusion parameters and nRMSE between both diffusion encoding schemes using Wilcoxon signed-rank test. A P value of < 0.05 was considered statistically significant.
Results and Discussion
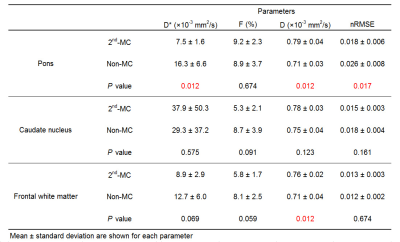
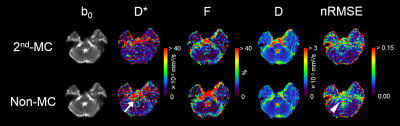
Table 1 shows the diffusion parameters and nRMSE obtained with the 2nd-MC and non-MC diffusion encoding. Examples of IVIM diffusion parameter and nRMSE maps for one representative subject are shown in Figure 2. The D* of the pons with non-MC was significantly higher than that with 2nd-MC, whereas there were no significant differences in the D* of the caudate nucleus and frontal white matter between both diffusion encoding schemes. A previous study has demonstrated the largest brain motion in the pons, which is more than 4-fold larger displacement than the frontal lobe.6 Thus, our results suggest that D* with non-MC is strongly affected by the bulk motion in the pons. Moreover, the bulk motion-induced signal loss in the pons, especially for low b-values, can be compensated by the 2nd-MC diffusion encoding, which results in lower D*. In the pons, the nRMSE significantly reduced when using the 2nd-MC, indicating improved accuracy of the biexponential fitting. We also found significant differences in the D of the pons and frontal white matter between the 2nd-MC and non-MC. This result might be contributed to the dependence of restricted diffusion on the difference in the diffusion encoding waveform between both schemes.Conclusion
The 2nd-MC diffusion encoding scheme reduces the bulk motion effect on IVIM analysis of the brain, thereby improving the measurement accuracy.Acknowledgements
No acknowledgement found.References
1. Iima M, Le Bihan D. Clinical Intravoxel Incoherent Motion and Diffusion MR Imaging: Past, Present, and Future. Radiology. 2016;278:13-32.
2. Wirestam R, Greitz D, Thomsen C, et al. Theoretical and experimental evaluation of phase-dispersion effects caused by brain motion in diffusion and perfusion MR imaging. J Magn Reson Imaging. 1996;6:348-355.
3. Nguyen C, Fan Z, Xie Y, et al. In vivo diffusion-tensor MRI of the human heart on a 3 tesla clinical scanner: An optimized second order (M2) motion compensated diffusion-preparation approach. Magn Reson Med. 2016;76:1354-1363.
4. Aliotta E, Moulin K, Magrath P, et al. Quantifying precision in cardiac diffusion tensor imaging with second-order motion-compensated convex optimized diffusion encoding. Magn Reson Med. 2018;80:1074-1087.
5. Lv J, Huang W, Zhang J, et al. Performance of U-net based pyramidal lucas-kanade registration on free-breathing multi-b-value diffusion MRI of the kidney. Br J Radiol. 2018;91:20170813.
6. Soellinger M, Ryf S, Boesiger P, et al. Assessment of human brain motion using CSPAMM. J Magn Reson Imaging. 2007;25:709-14.
Figures