4304
Diffusion tensor imaging in human subjects wearing metallic orthodontic braces1Neurosection, Division of MRI Research, Russell H. Morgan Department of Radiology and Radiological Science, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 2F.M. Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Institute, Baltimore, MD, United States, 3Department of Medical Imaging, Nanfang Hospital, Southern Medical University, Guangzhou, China, 4Johns Hopkins University School of Medicine, Division of Neuroradiology, Russell H. Morgan Department of Radiology and Radiological Science, Baltimore, MD, United States, 5Department of Neurosurgery, Johns Hopkins University School of Medicine, Baltimore, MD, United States
Synopsis
Metallic objects such as dental braces bring substantial susceptibility artifacts in MR images acquired using echo-planar-imaging (EPI) sequences. Here, we demonstrate that diffusion-prepared diffusion tensor imaging (DTI) with three-dimensional fast gradient-echo readout can significantly reduce susceptibility artifacts that are commonly seen in conventional spin-echo (SE) EPI DTI in the presence of metallic orthodontic braces.
Introduction
Dental fillings and orthodontic braces containing various metals can cause large susceptibility artifacts extending from the facial region into the brain1,2,3 in commonly used echo-planar imaging (EPI) based sequences. This is particularly a problem for diffusion-tensor-imaging (DTI) MRI studies4, where spin echo (SE)-EPI sequences are commonly used. Such susceptibility artifacts include signal dropout and geometric distortion in affected regions. SE-EPI in principle has less signal dropouts than gradient echo (GRE) EPI. However, geometric distortion due to local B0 inhomogeneity can still be significant in SE-EPI. Therefore, many DTI studies choose to acquire two identical DTI scans with opposite phase encoding directions in order to correct for such distortion, at the expense of doubling scan time and introducing potential motion and physiological variations between scans5. Alternatively, diffusion contrasts can be induced using spin preparation modules before readout6,7 which separates contrast generation from the readout, thereby opening the possibility to use virtually any pulse sequence for image acquisition. Among the various strategies, a double refocusing diffusion preparation module followed by a 3D readout has been commonly adopted8,9, which can significantly reduce image distortion commonly seen in EPI10. In this study, we applied a diffusion-prepared-DTI approach8,11 with single-shot 3D fast-gradient-echo (GRE) readout in healthy human subjects wearing metallic orthodontic braces to evaluate its ability to minimize susceptibility artifacts in the presence of metallic objects12.Methods
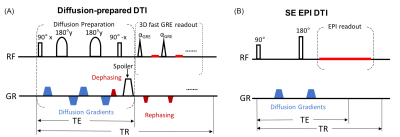
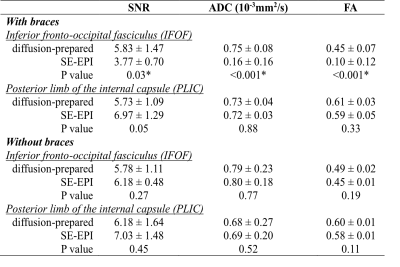
Six healthy participants (40±6yo, 3 females) were recruited for this study. Removable dental braces with bonding trays were used so that MRI images can be acquired with braces or without braces in the same participants. Figure 1 illustrates the DTI sequences. The diffusion-prepared DTI sequence includes a diffusion preparation module for generating the desired diffusion contrast, followed by a single-shot 3D fast GRE readout. The diffusion preparation module uses double refocusing pulses, with additional diffusion weighting gradients inserted between the RF pulses6,7,13,14. To minimize eddy current related artifacts and reduce T1 effects during the readout, a stimulated echo scheme8 is adopted: a dephasing gradient in the slice-encoding direction before the last 90° pulse in the diffusion preparation module, and a set of rephasing gradients in the readout (each of which has the same area as the dephasing gradient) are added. The following scans were acquired for each subject on a 3T Philips MRI scanner: MPRAGE (voxel=1x1x1mm3); SE-EPI DTI: b=0 and 800s/mm2, 15 diffusion gradient directions voxel=2.5x2.5x2.5mm3; diffusion-prepared DTI: same b-values, diffusion gradients and voxel size as SE-EPI DTI, TR/TE(effective)=5000/90ms, FA=11°, SENSE factor=3x1, centric order, TRGRE/TEGRE=4.1/2.1ms, dephasing gradient=2mT/m and 5ms. DTI data were processed using MRI-Studio (www.mristudio.org). Signal-to-noise-ratio (SNR), apparent-diffusion-coefficient (ADC) and fractional-anisotropy (FA) were compared in two manually drawn ROIs: bilateral inferior fronto-occipital fasciculus (IFOF) with strong susceptibility artifacts, and bilateral posterior limb of internal capsule (PLIC) with minimal susceptibility artifacts in EPI. Geometric distortion was visualized using Slicer in FSL (https://fsl.fmrib.ox.ac.uk/), and quantified by the Jaccard index, which ranges from zero to one, indicating no overlap to complete agreement, respectively, between the geometric shapes of the DTI image and reference structural images.Results
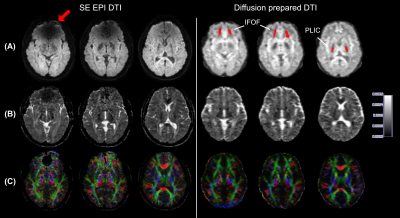
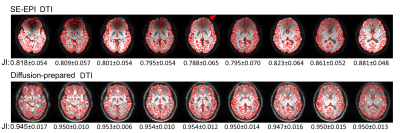
Figure 2 shows typical raw diffusion-weighted images, ADC, and color-coded FA maps from one subject wearing braces. While SE-EPI shows signal loss in many brain regions (e.g. the frontal lobe in the slice shown), no obvious artifacts were visible in the diffusion-prepared EPI in the entire brain. Color coded FA maps obtained from SE-EPI DTI showed spurious results in the inferior frontal lobe near the brace (red arrow in Figure 2), affecting visualization of the IFOF, as compared to diffusion-prepared color FA maps in the same subject. Geometric distortion (Figure 3) was minimal in diffusion-prepared DTI, but was substantial in SE-EPI DTI (significantly lower Jaccard index in each slice, P < .001), and the degree of distortion varied with the location of the slice. Table 1 summarizes the group-averaged quantitative results from all subjects from the ROI analysis (ROIs delineated in Figure 2). ADC, FA and SNR values were all comparable between diffusion-prepared and SE-EPI in the PLIC, a structure minimally affected by the susceptibility artifacts. In the IFOF, which is close to the dental braces, SNR was significantly diminished in SE-EPI, leading to erroneous ADC and FA values, whereas diffusion-prepared DTI showed greater SNR and reasonable ADC and FA values consistent with the literature16. When the same scans were repeated in the same subjects without wearing the metallic dental braces, ADC, FA and SNR in both the PLIC and the IFOF became comparable between SE-EPI and diffusion-prepared DTI scans, all of which are within the typical range reported in the literature.Discussion & Conclusion
We demonstrate that diffusion-prepared-DTI can acquire diffusion MR images in healthy human subjects wearing metallic dental braces with preserved SNR for the entire brain, whereas conventional SE-EPI DTI showed significantly reduced SNR in regions with strong susceptibility effects. Also, the geometric distortion was reduced significantly with diffusion-prepared DTI. This technique is expected to provide an alternative approach in studies involving regions with large susceptibility artifacts caused by metallic implants in the brain.Acknowledgements
NINDS (1R01NS108452), NIBIB (R21EB 023538 and P41 EB015909), NICHD (U54 HD079123).References
1. Starčuková J, Starčuk Z, Hubálková H, Linetskiy I. Magnetic susceptibility and electrical conductivity of metallic dental materials and their impact on MR imaging artifacts. Dent Mater. 2008;24(6):715-723. doi:10.1016/J.DENTAL.2007.07.002
2. New PF, Rosen BR, Brady TJ, et al. Potential hazards and artifacts of ferromagnetic and nonferromagnetic surgical and dental materials and devices in nuclear magnetic resonance imaging. Radiology. 1983;147(1):139-148. doi:10.1148/radiology.147.1.6828719
3. Hinshaw DB, Holshouser BA, Engstrom HI, Tjan AH, Christiansen EL, Catelli WF. Dental material artifacts on MR images. Radiology. 1988;166(3):777-779. doi:10.1148/radiology.166.3.3340777
4. Goto M, Abe O, Hata J, et al. Adverse effects of metallic artifacts on voxel-wise analysis and tract-based spatial statistics in diffusion tensor imaging. Acta radiol. 2017;58(2):211-217. doi:10.1177/0284185116641348
5. Sotiropoulos SN, Jbabdi S, Xu J, et al. Advances in diffusion MRI acquisition and processing in the Human Connectome Project. Neuroimage. 2013;80:125-143. doi:10.1016/J.NEUROIMAGE.2013.05.057 6. Lee H, Price RR. Diffusion imaging with the MP-rage sequence. J Magn Reson Imaging. 1994;4(6):837-842. doi:10.1002/jmri.1880040616
7. Sinha U, Sinha S. High speed diffusion imaging in the presence of eddy currents. J Magn Reson Imaging. 1996;6(4):657-666. doi:10.1002/jmri.1880060415
8. Zhang Q, Coolen BF, Versluis MJ, Strijkers GJ, Nederveen AJ. Diffusion-prepared stimulated-echo turbo spin echo (DPsti-TSE): An eddy current-insensitive sequence for three-dimensional high-resolution and undistorted diffusion-weighted imaging. NMR Biomed. 2017;(January):1-12. doi:10.1002/nbm.3719
9. Jeong E-K, Kim S-E, Parker DL. High-resolution diffusion-weighted 3D MRI, using diffusion-weighted driven-equilibrium (DW-DE) and multishot segmented 3D-SSFP without navigator echoes. Magn Reson Med. 2003;50(4):821-829. doi:10.1002/mrm.10593
10. Hiwatashi A, Togao O, Yamashita K, et al. Evaluation of diffusivity in pituitary adenoma: 3D turbo field echo with diffusion-sensitized driven-equilibrium preparation. Br J Radiol. 2016;89(1063):20150755. doi:10.1259/bjr.20150755
11. Hiwatashi A, Togao O, Yamashita K, et al. 3D turbo field echo with diffusion-sensitized drivenequilibrium preparation technique (DSDE-TFE) versus echo planar imaging in evaluation of diffusivity of retinoblastoma. Br J Radiol. 2016;89(1067):15-17. doi:10.1259/bjr.20160074
12. Miao X, Wu Y, Liu D, et al. Whole-brain functional and diffusion tensor mri in human participants with metallic orthodontic braces. Radiology. (in press)
13. Coremans J, Spanoghe M, Budinsky L, et al. A Comparison between Different Imaging Strategies for Diffusion Measurements with the Centric Phase-Encoded TurboFLASH Sequence. J Magn Reson. 1997;124(2):323-342. doi:10.1006/JMRE.1996.1025
14. Thomas DL, Pell GS, Lythgoe MF, Gadian DG, Ordidge RJ. A quantitative method for fast diffusion imaging using magnetization-prepared turboFLASH. Magn Reson Med. 1998;39(6):950-960. doi:10.1002/mrm.1910390613
15. Gibbs SJ, Carpenter TA, Hall LD. Diffusion imaging with unshielded gradients. J Magn Reson. 1992;98(1):183-191. doi:10.1016/0022-2364(92)90123-O 16. Mori S. Introduction to Diffusion Tensor Imaging. 1st ed. Elsevier; 2007.
16. Mori S. Introduction to Diffusion Tensor Imaging. 1st ed. Elsevier; 2007.
Figures