4301
SENSE accelerated multishot spiral diffusion: application in brain on a clinical platform1BIU MR, Philips Healthcare, Best, Netherlands, 2BIU MR, Philips Healthcare, Bangalore, India
Synopsis
In this study we compare SENSE accelerated multi-shot variable density spiral diffusion to the current clinical standards: single shot EPI and MultiVane TSE diffusion. A variable density sampling strategy was employed to correct for the phase of the different shots and iterative SENSE was used to reduce the number of shots and scanning duration. This technique was applied on a clinical platform with clinically acceptable reconstruction times. We showed that spiral diffusion reduces distortions in difficult to shim brain regions compared to ssh-EPI, and spiral diffusion has at a reduced scan duration compared to the TSE-based approach.
Introduction
Diffusion weighted imaging (DWI) commonly uses a single shot (ssh) echo planar imaging (EPI) readout preceded by diffusion sensitizing gradients. The advantage is that this is a very efficient technique, resulting in a short scan duration. However, due to the low imaging bandwidth ssh-EPI is highly sensitive to inhomogeneous magnetic fields resulting in geometric distortions. For anatomies or regions with a homogeneous magnetic field this is typically not an issue, however in more challenging areas, such as e.g. spine, brain stem, or body imaging ssh-EPI-based diffusion results in severely distorted images, which can limit the application in e.g. oncology(1). Using magnetic field inhomogeneity information can correct for the distortion(2), but areas of very strong magnetic field variations remain non-diagnostic. A possible alternative is to use a turbo SE (TSE) readout, which is geometrically accurate but is suffering from a lower SNR, or much longer scan duration. In this study we have implemented a variable density multi-shot spiral acquisition(3,4) to overcome the drawbacks of a low imaging bandwidth, while having a moderate scan duration increase, due to the high efficiency of a spiral readout(5–7). This technique is compared against the most commonly used other techniques, ssh-EPI DWI and MultiVane (MV) TSE DWI.Methods
After obtaining informed consent, 4 healthy subjects underwent three DWI sequences on a standard Philips Ingenia Elition 3T platform (Philips Healthcare, Best, The Netherlands). 1. DWI ssh-EPI, 2. DWI spiral and 3. MV TSE DWI all with identical resolution and coverage: 1.5mmx1.5mmx5mm, 24 slices and max b-factor 1000 s/mm2. For the spiral acquisition, a spiral out variable density scheme was employed with a fully sampled center k-space radius of 10%, which was used to apply intra-shot phase correction. The acquisition used the standard gradient corrections implemented on the clinical system. No additional gradient adjustments or eddy corrections were needed or applied. Reconstruction was performed online on the standard clinical system and included deblurring based on a B0 field map , iterative SENSE and intra-shot phase correction. An overview of parameters is given in table 1. The DWI ssh-EPI was reconstructed twice, with and without EPI geometry correction (using a B0-map and reversed phase encoding direction).Results & Discussion
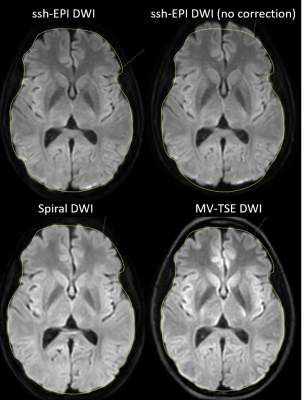
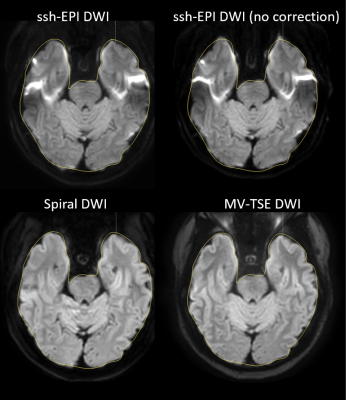
Figure 1 shows the comparison between the different acquisition methods for a slice through the center of the brain of the b1000 image. The results clearly show the distortion as expected on the non-corrected ssh-EPI acquisition (top right), which can be corrected at this location using EPI geometry correction. The multishot spiral DWI matches the true anatomy better, when comparing to the TSE DWI acquisition. The outline of the slice is measured on the TSE-DWI image and copied to the other acquisitions. Figure 2 shows the same comparison for a different subject and at a lower slice location. At this level where strong magnetic field inhomogeneities are present, the ssh-EPI acquisition shows pronounced distortions and areas of signal void and pile-up. The overall shape can be recovered using EPI geometry correction, however it cannot correct for the signal voids and signal pile-up. Multishot Spiral DWI matches the anatomy of the MV-TSE DWI acquisition and does not suffer from areas of signal loss or pile-up, similarly to the MV-TSE DWI acquisition, however at only half the scan duration (2:08min vs. 5:10min, for spiral-DWI and TSE-DWI, respectively). The image deblurring algorithm used in the spiral reconstruction can remove the majority of spatial blurring, however some residual blurring is visible in figure 2, at the region of the temporal lobes which exhibits strong spatial magnetic field inhomogeneities. This effect is estimated to be much less detrimental in clinical imaging than the signal voids present at the EPI acquisition. Recon latencies are larger using Spiral DWI but still within acceptable ranges and are expected to reduce further with the introduction of more powerful processing hardware.Conclusion
Multishot spiral DWI is much less sensitive to areas in the brain that exhibit high magnetic field inhomogeneities than ssh EPI based approaches. Even if geometry correction is applied, ssh EPI still suffers from signal voids and pile-up. MultiVane TSE DWI has a high anatomical accuracy, however at the cost of a prolonged scan time. Spiral DWI is more robust to distortions than ssh-EPI and is more than twice as fast than MV-TSE DWI acquisition. The combination of a variable density spiral acquisition scheme, with intra-shot phase correction and iterative sense was implemented on a clinical 3T platform and is compatible with the clinical standard workflow.Acknowledgements
No acknowledgement found.References
1. White NS, Cancer Res. 2014;74:4638–4652 doi: 10.1158/0008-5472.CAN-13-3534. 2.Andersson, NeuroImage, 20(2):870-888, 2003. 3. Holtrop JL, J. Med. Imaging Bellingham Wash 2016;3:023501 doi: 10.1117/1.JMI.3.2.023501. 4. Liao C, Magn. Reson. Med. 2017;77:1359–1366 doi: 10.1002/mrm.26199. 5. Börnert P, Magma N. Y. N 1999;9:29–41 doi: 10.1007/bf02634590. 6. Li Z, Am. J. Neuroradiol. 2016;37:642–647 doi: 10.3174/ajnr.A4600. 7. Li Z, Magn. Reson. Med. 2020;83:170–177 doi: 10.1002/mrm.27911.
Figures