4292
Measuring cardiac activity with a thermal noise based self-navigator1Radiotherapy, UMC Utrecht, Utrecht, Netherlands, 2Computational Imaging Group for MRI Diagnostics & Therapy, Center for Image Sciences, UMC Utrecht, Utrecht, Netherlands, 3Research and Practical Clinical Center of Diagnostics and Telemedicine Technologies of the Moscow Department of Healthcare, Moscow, Russian Federation
Synopsis
The ECG signal can be disturbed by the magnetohydrodynamic effect and requires placement of ECG electrodes. The latter adds extra workload in the patient setup. Moreover, for MRI-guided radiotherapy, ECG skin electrodes will interfere with dose delivery and thus cannot be used. We propose to use the noise navigator, a passive self-navigation method based on the physiological motion-induced modulation of the thermal noise variance measured by an RF receive coil, which does not require additional hardware and is inherently synchronized with MR acquisition.
Introduction
The beating heart and breathing are the principle (semi)periodic sources of physiological motion. Currently, the ECG is the best way to monitor cardiac activity (for e.g. cardiac MRI synchronisation). The ECG signal, however, can be disturbed by the magnetohydrodynamic effect1 and requires placement of ECG electrodes. The latter adds extra workload in the patient setup. Moreover, for MRI-guided radiotherapy, ECG skin electrodes will interfere with dose delivery and thus cannot be used. We propose to use the noise navigator, a passive self-navigation method based on the physiological motion-induced modulation of the thermal noise variance measured by an RF receive coil2, which does not require additional hardware and is inherently synchronized with MR acquisition. We have previously demonstrated the application of the noise navigation for respiration detection2, here we investigated its applicability for cardiac activity detection as inspired by our previous electromagnetic simulation study3. For MRI-guided radiotherapy, the noise navigator was combined with a radiolucent high-impedance coil4. The coil position was optimized on a single volunteer. After that, cardiac activity detection experiments were performed on five healthy volunteers on a clinical 1.5T MRI system and the ECG signal was recorded simultaneously for validation.Methods
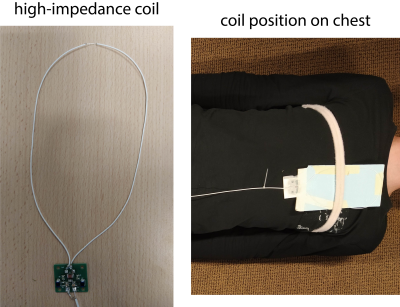
All measurements were performed with a single high-impedance coil4. To investigate the effect of the position of the coil with respect to the heart on the sensitivity to detect cardiac activity, measurements during breath hold were performed with a network analyzer. This active measurement represents the electromagnetic reciprocal counterpart of passive thermal noise detection3. The real part of the effective impedance of the coil, which has a linear relationship with the noise navigator, was calculated from the complex reflection coefficient (i.e. S11).Noise-only experiments were performed on five healthy volunteers (three male and two female) on a clinical 1.5T MR scanner. These noise-only acquisitions were measured with a balanced gradient echo sequence (2.47 ms TR and 1 MHz receive bandwidth) where the gradients and RF excitation were turned off. The coil was fixated on the volunteer at 2 cm to the left of the sternum using a velcro strap (see Figure 1). Two breath hold (18 s) and two free breathing (60 s) experiments were performed per volunteer. The ECG was simultaneously acquired with four electrodes positioned according to clinical protocol as a reference method for cardiac activity.A Kalman filter was applied to the measured thermal noise variance, to extract the cardiac activity between 0.7 and 1.4 Hz (i.e. 42 and 84 beats per minute) and breathing4.
Results
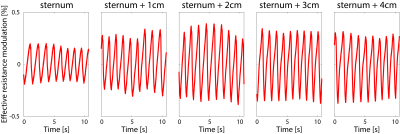
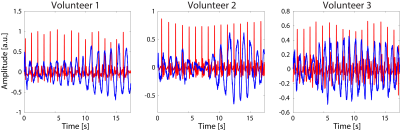
The highest effective resistance modulation (i.e. 0.65$$$\%$$$), and thus optimal coil position with respect to the heart, was observed at 2 cm to the left of the sternum (see Figure 2).An example of the noise navigator compared to the ECG is shown in Figure 3 for a single breath hold experiment for each of the three male volunteers. Cardiac activity could not be detected with the noise navigator for the female volunteers.
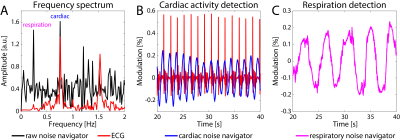
In Figure 4A the cardiac and respiratory motion can be seen in the unfiltered noise navigator frequency spectrum at 0.76 and 0.24 Hz, respectively. Cardiac and respiratory motion were extracted from the same free breathing experiment on a healthy male volunteer by applying two separate parallel Kalman filters. A good match between the cardiac noise navigator and ECG signal was observed (see Figure 4B).
Discussion & Conclusion
It was feasible to detect cardiac activity during breath hold in all three males, but failed in both female volunteers. It is hypothesized that a lower cardiac activity imprint in the noise navigator is caused by the larger distance between the coil and the heart due to breast tissue for females3. As the setup was optimized on a male volunteer, repeating this for a female could yield improved results.During free breathing, cardiac activity could be detected in two out of three male volunteers. In the third volunteer only respiration was visible. Based on these initial results, we believe that cardiac activity detection with the noise navigator is possible. Currently, however, interference between respiration and cardiac induced modulation in thermal noise is observed causing amplitude variations and some residual timing variations between noise navigator and the ECG signal. We believe that improved processing (e.g. using a recurrent neural network) and further optimized acquisition setup (e.g. exploiting other receive array elements distant from the heart that are only sensitive to respiration) could enhance the separation between cardiac and respiration induced modulations. Moreover, the additional information provided by the noise covariances of receive array elements close to the heart will be investigated.
Here we showed the cardiac activity detection feasibility with a single high-impedance coil. The noise navigator, however, is not restricted to this type of coil. The experiment at the MR scanner was repeated on one of the male volunteers with a clinical 10 cm diameter loop coil and cardiac activity was observed in this case too.
The feasibility of cardiac motion detection was shown with noise-only measurements, but cardiac activity detection through the noise navigator simultaneously with MR image acquisition should be possible and will be investigated in the future.
Acknowledgements
No acknowledgement found.References
[1] M. Jekic, Y. Ding, R. Dzwonczyk, P. Burns, S.V. Raman, and O.P. Simon-etti, “Magnetic field threshold for accurate electrocardiography in the MRIenvironment,” Magnetic Resonance in Medicine, vol. 64, no. 6, pp. 1586–1591, 2010
[2] R.J.M. Navest, A. Andreychenko, J.J.W. Lagendijk, and C.A.T. van denBerg, “Prospective Respiration Detection in Magnetic Resonance Imaging bya Non-Interfering Noise Navigator,” IEEE Transactions on Medical Imaging,vol. 37, pp. 1751–1760, aug 2018
[3] R.J.M. Navest, S. Mandija, A. Andreychenko, A.J.E. Raaijmakers,J.J.W. Lagendijk, and C.A.T. Berg, “Understanding the physical relations governing the noise navigator,” Magnetic Resonance in Medicine,vol. 82, pp. 2236–2247, dec 2019
[4] S.E. Zijlema, R.H.N. Tijssen, V.N. Malkov, L. van Dijk, S.L. Hackett,J.G.M. Kok, J.J.W. Lagendijk, and C.A.T. van den Berg, “Design andfeasibility of a flexible, on-body, high impedance coil receive array for a 1.5T MR-linac,” Physics in Medicine & Biology, vol. 64, p. 185004, sep 2019
Figures