4284
Line scan-based dynamic imaging of fluid motion periodically driven by MR actuator1Center for Neuroscience Imaging Research, Institute for Basic Science, Suwon, Republic of Korea, 2Dept. of Biomedical Engineering, Sungkyunkwan University, Suwon, Korea, Republic of, 3Dept. of Physics, Sungkyunkwan University, Suwon, Korea, Republic of
Synopsis
Imaging fluid motion in MRI is possible with high temporal resolution and reliable motion-inducing mechanism. In this study, line-scanning with 5.5 ms temporal resolution was used to image fluid motion inside quail eggs which were periodically driven by an MR actuator. Resulting time-course images captured accurate displacements of the eggs and the internal fluid motion over time.
Introduction
Line-scanning enables collection of sequential images in high temporal resolution by acquiring one k-space line within each short repetition.1-4 This can be particularly useful for imaging rapidly moving objects, such as fluids in motion. Previous publications have shown applications of magnetic resonance imaging (MRI) in complex fluid imaging.5 This study examines the possibility of using line-scanning to image fluid motion which is mechanically driven by an MR actuator.Methods
MR ActuatorAn MR-actuator was constructed using a 15 cm-long 3D-printed structure (actuator arm) and an insulated copper wire (0.15 mm in diameter without insulation). The wire was wound around square-shaped wire holders (3.5 × 3.5 cm2) 23 times on one end of the actuator. On the opposite end of the actuator, an object to be scanned (quail egg) was placed on a holding surface and fixed with tape. The protruded hinge of the arm was fitted to a custom cradle and inserted into the MRI scanner, as shown in Figure 1.
The wire loop was connected to a 10Ω resistor in series, and a function generator which provided varying voltage output in sinusoidal waves, ranging between -5V and +5V. As a result, the current loop formed in the wire windings produced a magnetic moment, μ, normal to the square surface of the loop. Subsequently, torque was exerted on the actuator arm as a result of the relationship τ = μ × B0, where B0 is the main magnetic field of the MRI scanner. The torque was adjusted to rotate the actuator arm up to 17.5 degrees in clockwise and counter-clockwise directions, through remote control of the polarity and magnitude of the function generator's voltage output from the operator room.
Imaging with Line-scanning Sequence
A 9.4-T Bruker scanner (BioSpec 94/30, Ettlingen, Germany) was used to scan the quail eggs in motion. A fast low-angle shot (FLASH) line-scan sequence developed by Lee, et al. (2018) was used with the following parameters: TR/TE = 5.5/2.502 ms, 9° flip angle, 2 mm slice-thickness, 128 × 128 matrix size, and 55 × 55 mm2 FOV.1
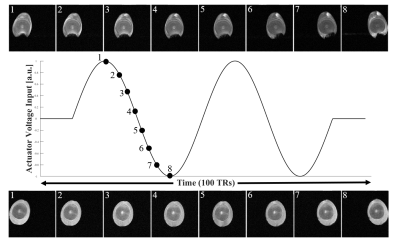
The scanner system triggered the function generator every 100-TRs, prompting it to produce two periods of sinusoidal wave with a ±5 V amplitude. One k-space line per image was acquired with the following trigger sequence: 10/80/10-TR off/on/off. During the "on" period, 2 sinusoidally varying voltage outputs were delivered to the actuator. This sequence was repeated until all 128 k-space lines were acquired per image. Total 100 2D time-course images were collected and reconstructed.
Results
While the k-space lines of each 2D reconstructed image were acquired 100-TRs away from one another, highly repetitive motion generated by the actuator produced images which accurately represent quail eggs' displacements over time. Figure 2 shows distinctive differences between raw and cooked quail eggs in motion. Small air cell located beneath the raw egg's yolk can be observed in the figure. The fluid motion within the shell was denoted by the changing shape of the air cell which was imaged at each time point. On the contrary, images of cooked egg showed no sign of fluid motion.Conclusion
This study shows 2D line-scanning can be employed with high temporal resolution (5.5 ms) to image fluids in motion. An MR actuator can mechanically drive such motion with highly configurable movements. In comparison, MR elastography (MRE) uses acoustic vibrations and phase imaging to measure wave propagations in solid tissues and assesses tissue stiffness in human organs.6 Further experiments involving fluid imaging with methods similar to what is described in this study have a potential to measure wave propagations in fluids and non-invasively measure their properties.Acknowledgements
This work was supported by the Institute for Basic Science (IBS-R015-D1) and National Research Foundation of Korea (NRF-2019M3C7A1031993).References
1. J. Lee, S.-K. Lee, and J.-Y. Park, "A novel method for direct detection and spatial mapping of neuronal activity." Presented at: 26th Annual Meeting of International Society for Magnetic Resonance in Medicine, Paris, France,June 2018.
2. F. Albers, F. Schmid, L. Wachsmuth, and C. Faber, "Line scanning fmri reveals earlier onset of optogenetically evoked bold response in rat somatosensory cortex as compared to sensory stimulation," Neuroimage, vol. 164, pp.144-154, 2018.
3. T. N. Kim, P. W. Goodwill, Y. Chen, S. M. Conolly, C. B. Schaffer, D. Liepmann,and R. A. Wang, "Line-scanning particle image velocimetry: an optical approach for quantifying a wide range of blood ow speeds in liveanimals," PloS one, vol. 7, no. 6, p. e38590, 2012.
4. T. Phan, H. Lee, J. T. Lee, S.-K. Lee, and J.-Y. Park, "Progress towards direct in-vivo detection and mapping of neuronal activity." Presented at:27th Annual Meeting of International Society for Magnetic Resonance in Medicine, Montreal, Canada, May 2019.
5. D. Bonn, S. Rodts, M. Groenink, S. Rafaï, N. Shahidzadeh-Bonn, and P. Coussot, "Some applications of magnetic resonance imaging in fluid mechanics: Complex flows and complex fluids," Annual Review of Fluid Mechanics, vol. 40, no. 1, pp. 209-233, Jan. 2008. [Online]. Available:https://doi.org/10.1146/annurev.fluid.40.111406.102211
6. S. K. Venkatesh, M. Yin, and R. L. Ehman, "Magnetic resonance elastography of liver: Technique, analysis, and clinical applications," Journal of Magnetic Resonance Imaging, vol. 37, no. 3, pp. 544{555, Feb.2013. [Online]. Available: https://doi.org/10.1002/jmri.23731
Figures