4277
High resolved 13C MRS of myo-Inositol C1-C6 in the human brain in vivo at 7T1Laboratory of Functional and Metabolic Imaging (LIFMET) - Ecole Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland, 2Centre d'Imagerie BioMédicale - Animal and Imaging Technology (CIBM-AIT) - Ecole Polytechnique de Lausanne (EPFL), Lausanne, Switzerland, 3Department of Radiology, Universities of Lausanne (UNIL) and Geneva (UNIGE), Lausanne, Switzerland
Synopsis
The quantification of metabolite signals in the 1H-spectrum of the human brain in vivo is complicated by an extensive spectral overlap of resonances, due to the restricted chemical shift dispersion of the 1H-nucleus. For instance, the myo-Inositol peaks observable in the 1H-spectrum at 7T are close the water resonance and overlap with that of glycine. In contrast, the high resolution of the 13C-MR spectrum aids in the interpretation of the highly sensitive but poorly resolved 1H-MR spectrum. In this study, we demonstrate the advantage of 13C-MRS at 7T by measuring high resolved myo-Inositol C1-C6 resonances in the human brain.
Introduction
In vivo 13C-MRS at ultra-high field (i.e. ≥ 7T) benefits significantly from improved signal-to-noise ratio (SNR) and spectral resolution for the resolution of overlapped metabolite signals. Precisely, the large chemical shift dispersion of the 13C-nucleus makes in vivo 13C-MRS potentially useful for quantitative measurements of metabolites that are difficult to resolve in the 1H-spectrum, such as myo-Inositol [1]. An active transport through the blood-brain barrier maintains myo-Inositol in the brain at much higher concentration than in blood, predominantly in central cerebellum and occipital cortex [2]. However, whilst owning six equivalent CH protons, quantitative measurements of myo-Inositol by in vivo 1H-MRS up to 7T have been limited to two resonances (H1,3 and H4,6) [3], due to the restricted chemical shift dispersion of the 1H-nucleus. Because of the inherent low sensitivity of the 13C-nucleus and its low natural abundance, in vivo 13C-MRS is typically performed using surface coils, as their high filling factor results in increased SNR, if using adiabatic pulses [4]. In addition, optimal sensitivity and spectral resolution of 13C-MRS requires efficient 1H-decoupling, which can be achieved with low transmit power using a 1H-quadrature RF coil [5]. In this context, the aim of this study was to explore the advantage of 13C-MRS at 7T in terms of sensitivity gain and improved spectral resolution, and to investigate its potential feasibility towards quantitative measurements of myo-Inositol C1-C6 in the human brain in vivo at 7T.Methods
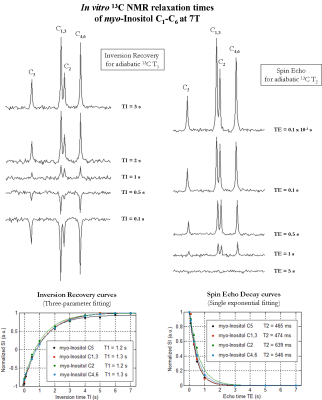
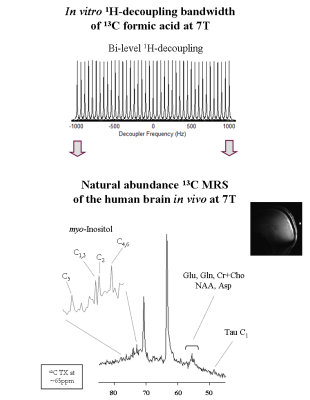
All measurements were performed on a 7T human scanner (Siemens Erlangen/Germany) using a home-built 13C-linear/1H-quadrature RF surface coil. A pulse-acquire sequence for sensitivity-enhanced 13C-MRS using bi-level 1H-decoupling was developed. Uniform 13C-excitation was achieved at 7T using two phase-inverted adiabatic half-passage (AHP) pulses of 2ms applied in alternate scans [6] within 650Hz to cover the ~3.2ppm of myo-Inositol C1-C6. Bi-level 1H-decoupling consisted of a series of equidistant WALTZ-cycles interleaved with time delays during 13C-relaxation time for the generation of low-power NOE, and WALTZ-16 [7] for broadband 1H-decoupling during 13C-signal acquisition. The duration of the main WALTZ-cycle 90°-pulse was fixed to 1.5ms to cover the ~1ppm of myo-Inositol H1-H6. The 13C-MR relaxation properties of myo-Inositol C1-C6 at 7T were evaluated in vitro on a phantom containing 150mM of myo-Inositol. In particular, the NOE enhancement was measured by applying successively a series of WALTZ-cycles until the steady-state was reached. In addition, the spin-lattice (13C-T1) and spin-spin (13C-T2) relaxations times were measured using an optimized adiabatic inversion recovery and Hahn spin-echo [8] sequences. All in vitro 13C-MRS measurements were performed using the centre of the 13C-spectrum at the C1,3 resonance (i.e. 73.3ppm), while high spectral resolution was achieved using the appropriate acquisition time and decoupling duration based on the T2* calculated from the natural line-width of the myo-Inositol C1-C6 resonances at 7T. Natural abundance 13C-MR spectra were acquired in vivo from the occipital cortex of two healthy volunteers who signed informed consent of the local ethics committee. First and second order shims were adjusted using FAST(EST)MAP [9].Results and discussion
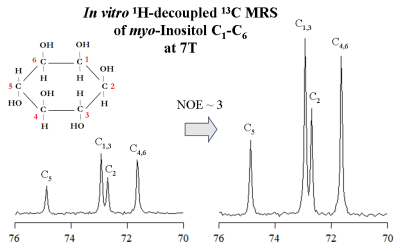
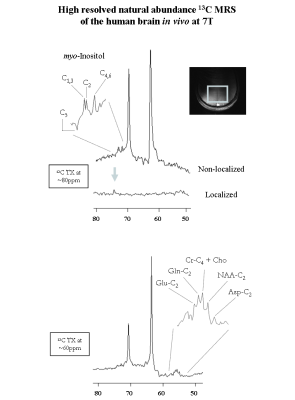
In vitro 1H-decoupled 13C-MRS of myo-Inositol at 7T revealed high spectral resolution including two degenerate resonances at 73.3ppm (C1 and C3) and 71.9ppm (C4 and C6) and two single resonances at 75.1ppm (C5) and 73.1ppm (C2) (Figure1 left). In addition, improved sensitivity was implied using bi-level 1H-decoupling by a maximal NOE enhancement factor of 3, achieved by increasing the TR (Figure1 right). Besides, the 13C-T1 of distinct myo-Inositol carbons were found to be rather similar to each other (Figure2 left), while the 13C-T2 were slightly disparate (Figure2 right). The natural line-width of all myo-Inositol carbons measured 7.5Hz, yielding to 42ms T2*. High spectral resolution was achieved by adjusting the acquisition time and decoupling duration to the FID (~215ms). The un-localized 13C-MR spectra acquired from the occipital cortex of the human brain in vivo at 7T revealed high resolved myo-Inositol C1-C6 resonances (Figure3 top), while the proximity of the dominating glycerol C2 peak (69.5ppm) induces a distortion in the baseline of the spectrum, which may complicate myo-Inositol quantification. A localized 13C-MR spectrum was acquired from the same subject and the two glycerol lipid peaks were completely suppressed, suggesting possible extension into localized myo-Inositol by increasing the scan time. In addition, by placing the excitation pulse downfield, several resonances were observed, which we tentatively assigned to Glutamate-C2 (55.6ppm), Glutamine-C2 (55.1ppm), Creatine-C4 and Choline (54.7ppm), N-acetyl-aspartate NAA-C2 (54ppm) and Aspartate-C2 (53.2ppm) (Figure 3 bottom). Simultaneous detection of myo-Inositol, metabolites from 53-56ppm above-mentioned, and the low-concentrated Taurine-C1 (48.4ppm) was possible by placing the excitation pulse in the middle of the spectrum in conjunction with bi-level broadband 1H-decoupling (Figure4).Conclusion
We conclude that 13C-MRS at 7T has the potential to provide high resolved myo-Inositol C1-C6 in the human brain in vivo, and that it could become the method of choice for investigating non-region specific brain diseases. The reduced baseline distortion using localization suggests possible extension into reliable quantification of myo-Inositol in the human brain in vivo at 7T. Although the measurement has been performed in the occipital cortex, it could be extended into the cerebellum for functional studies using a double-quadrature 13C/1H surface coil [10] that provides high sensitivity and better coverage of this brain region.Acknowledgements
This study was supported by Centre d’Imagerie BioMédicale (CIBM) of the UNIL, UNIGE, HUG, CHUV, EPFL and the Leenaards and Jeantet Foundations.
References
[1] Tkac I et al. MRM 2001;46:451-456
[2] Pouwels PJW et al. MRM 1998;39:53-60
[3] Scholz P et al. 1990 M. in Inos. Research
[4] Garwood M et al. JMR 2019;291:84-93
[5] Adriany G et al. MRM 1997;125:178-184
[6] Serés Roig E et al. NMR Biomed. 2019
[7] Shaka AJ. et al. JMR 1983;52:335–338
[8] Hahn EL Phys. Rev. 1950;80,580
[9] Gruetter R et al. MRM 2000;43,319-323
[10] Serés Roig E et al. MRM 2015;73:894-900
Figures