4275
Two-Channel, Stretchable, and Flexible Breast Coil Array1Weldon School of Biomedical Engineering, Purdue University, West Lafayette, IN, United States, 2Basic Medical Sciences, Purdue University, West Lafayette, IN, United States, 3School of Electrical & Computer Engineering, Purdue University, West Lafayette, IN, United States
Synopsis
Given the decrease in mammography sensitivity for those with denser breast tissue, MRI is often utilized for supplemental screening. Here we present a stretchable, flexible, two-channel breast RF coil array that allows for a customized fit to the breast and closest possible positioning to the skin. This coil array was created by utilizing conductive thread, stitched onto polyester/spandex blend fabric. Clear images of both the phantom and in-vivo were obtained. Omnidirectional stretching provided increased patient comfort and customized fit. With this close proximity, greater sensitivity can be achieved when compared to traditional breast coils.
Introduction
While mammography has been the conventional method for detecting breast cancer, the sensitivity of this imaging method decreases for those with denser breast tissue1,2. Supplemental screening using Magnetic Resonance Imaging (MRI) is recommended to those within this category and those with greater susceptibility to developing breast cancer 3,4. Among clinical imaging modalities, MRI has the highest sensitivity for breast cancer detection and is used to estimate tumor size and location prior to surgery3. Unlike mammography, MRI does not expose the patient to any radiation and does not require uncomfortable compression of the breast tissue against a hard-surfaced film. The current breast MRI method allows for the patient to lie prone with their breasts uncompressed in the breast radiofrequency (RF) coil, while padding is provided to support the head and lower torso. In order to achieve greater image resolution and provide a more comfortable breast exam, we present a stretchable, flexible, two-channel breast RF coil array that allows for a customized fit to the breast and closest possible positioning to the skin. This coil array was created by utilizing conductive thread stitched onto polyester/spandex blend fabric. Because the coil material can be anchored to a sports bra/fitted top, this coil allows a more comfortable fit on the patient.Methods
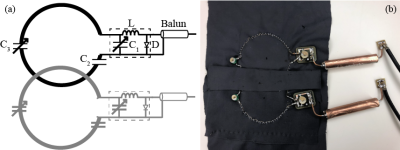
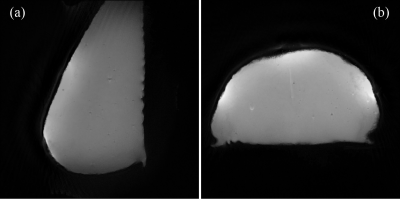
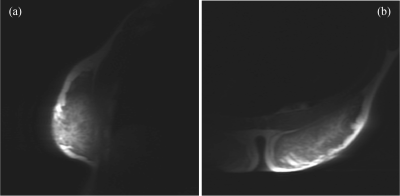
Lyofil (Syscom Advanced Material, Columbus, Ohio) fiber was zigzag stitched into two circles, roughly 71 mm in diameter, on a stretchable fabric (composition: 90% polyester and 10% spandex). The zigzag stretch stitch was anchored with a 100% polyester bobbin thread, using a Brother JX517 sewing machine (Brother International Corporation, Bridgewater, NJ). These coils were then hand-stitched together with an overlap spacing of 54.8 mm between the center of the two circular coils. This distance was determined by spacing the two centers of the circular loops 0.75 × diameter of the coil5. Due to the size variability, the diameters of the two coils were averaged for this calculation. Electrical components included a match/tune board, with an integrated current trap, and balun connected between the circuitry and coaxial cable. The coil array and circuitry are pictured in Fig. 1. All traps and coils were tuned 127.74 MHz, the Larmor frequency of hydrogen at 3T, using a vector network analyzer (E5071C, Keysight Technologies, Inc., Santa Rosa, California). Trap tuning was performed with a DC power supply at 5V to forward bias the diode and circuit. Then, matching and tuning were verified at -5V. Coil array scanning was completed on a 3T whole-body MR scanner (Discovery MR750, GE Healthcare, Chicago, IL). The array was connected to receiver gateway box (16xRx, Clinical MR Solutions, Brookfield, WI) using channels 1 and 2. Phantom scanning was performed on a SynAtomy complex breast phantom (SynDaver Labs, Tampa, FL). The coil was wrapped on top of the phantom, with the center placed on the horizontal breast apex, as shown in Fig. 2a. The scan protocol consisted of T2 and T1-weighted fast spin-echo (FSE) sequences with echo times (TE) of 131.248 ms (T2) and 4.28 (T1), repetition times (TR) of 4878 (T2) and 216 (T1), slice thicknesses of 5 mm (T2) and 3 mm (T1), pixel sizes of 0.23 mm × 0.23 mm (T2) and 0.47 mm × 0.47mm (T1), and grid of sizes 512 × 512 (T2) and 256 × 256 (T1). Scan parameters varied slightly for in vivo imaging, all reported images are T1-weighted with TE: 80.25, TR: 754.268, pixel size of 0.86 mm × 0.86 mm, slice thickness: 10 mm, and grid size of 512 × 512. The positioning of the coil was the same, as shown in Fig. 2b. The experimental procedures described in this abstract were approved by the local Institutional Review Board (protocol 19030219). The built-in scanner body coil was used to transmit, while the array was receive-only. Scan data was imported into MATLAB (Mathworks, Natick, MA) and SNR was calculated using an in-house code following the NEMA standards6.Results
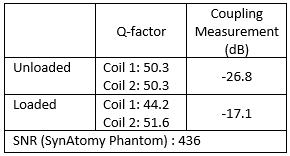
Quality factors were calculated using the method detailed by Doty et al.7 and are summarized in Fig. 3. SNR calculations were completed following the standard prescribed by NEMA6,8. These values are summarized along with the S21 coupling measurements in Fig. 3. Fig. 4 shows the single-slice images acquired from the FSE sequences in sagittal and axial views for the SynAtomy phantom. In vivo, single-single slice images acquired from the same sequences can be seen in Fig. 5.Discussion
The two-channel array coil offered an advantage in proximity of placement to the skin, as compared to traditional volume coils. The subject was comfortably fitted with the coil without needing to fully expose their breasts. Clear images of both the phantom and in-vivo were obtained. Slight artifacts are seen in the phantom images at the center of the coil overlap where water was retained in the phantom from storage. Motion artifacts were present in the in vivo scans, thus image clarity could be improved using respiratory gating.Conclusion
This omnidirectionally stretching coil array can provide increased patient comfort and customized fit across a variety of breast sizes. With close proximity, greater sensitivity can be achieved when compared to traditional breast coils. These results justify continuation of this project into a larger array.Acknowledgements
The authors would like to thank Antonia Susnjar for her assistance with MR scan setup and imaging.References
1. Siu, A. L. & Force, U. S. P. S. T. Screening for Breast Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med 164, 279-296, doi:10.7326/M15-2886 (2016).
2. von Euler-Chelpin, M., Lillholm, M., Vejborg, I., Nielsen, M. & Lynge, E. Sensitivity of screening mammography by density and texture: a cohort study from a population-based screening program in Denmark. Breast Cancer Res 21, 111, doi:10.1186/s13058-019-1203-3 (2019).
3. Mann, R. M., Cho, N. & Moy, L. Breast MRI: State of the Art. Radiology 292, 520-536, doi:10.1148/radiol.2019182947 (2019).
4. Vreemann, S. et al. The added value of mammography in different age-groups of women with and without BRCA mutation screened with breast MRI. Breast Cancer Res 20, 84, doi:10.1186/s13058-018-1019-6 (2018).
5. Roemer, P. B., Edelstein, W. A., Hayes, C. E., Souza, S. P. & Mueller, O. M. The NMR phased array. Magnetic Resonance in Medicine 16, 192-225, doi:10.1002/mrm.1910160203 (1990).
6. NEMA MR Standards: MS 6-2008 (R2014). (Rosslyn, VA: National Electrical Manufacturers Association, 2015).
7. Doty, F. D., Connick, T. J., Ni, X. Z. & Clingan, M. N. Noise in high-power, high-frequency double-tuned probes. Journal of Magnetic Resonance (1969) 77, 536-549, doi:10.1016/0022-2364(88)90011-x (1988).
8. NEMA MR Standards: MS 9-2008 (R2014). (Rosslyn, VA: National Electrical Manufacturers Association, 2015).
Figures