4253
RF-coil with variable resonant frequency for multiheteronuclear MRI1Faculty of Physics and Engineering, ITMO University, Saint-Petersburg, Russian Federation
Synopsis
We propose a double-coil setup for broad-range heteronuclear MRI. The system designed for an 11.7T scanner comprises two independent coils, one of them tuned to 1H frequency to perform anatomical imaging, and another one, wire-array coil, tuned to the X-nucleus (including 2H, 11B, 13C, 23Na, 7Li and 31P) frequency. In order to tune the X-coil over such a wide range, both structural capacitance and inductance of the coil are made variable; narrow range tuning of the 1H coil is achieved via conventional tuning-matching circuit. The design principle and setup tunability are investigated in simulations and experimentally.
In MRI hydrogen nuclei (commonly referred to as protons) are most often used to generate a detectable RF-signal. In addition to hydrogen, several other nuclei present in living organisms provide the magnetic resonance response. Heteronuclear MR measurements, i.e., experiments using non-proton nuclei, termed X-nuclei, offer information which is complementary to that of proton MRI1–4. A particular problem with conventional coil design for heteronuclear imaging is the low tunability range of the X-nucleus coil. This manifests in the coils being produced for single- or dual-nuclei use only, thus severely limiting the applicability range of a particular coil. This problem escalates with the B0 growth as the absolute frequency gap between the Larmor frequencies of two chosen nuclei increases.
Methods
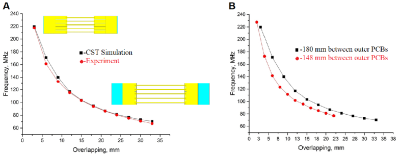
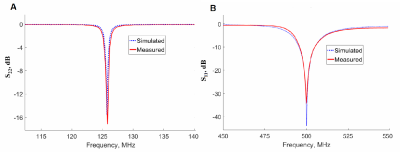
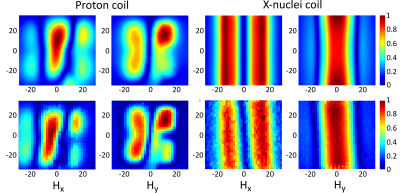
We propose a double-coil setup to allow broad range heteronuclear experiments: two independent coils, one of them tuned to 1H frequency to obtain anatomical reference images, and another, a wire-array coil5,6, tunable to a broad range of X-nuclei frequency. 2H and 31P frequencies were chosen as margin frequencies for the X-nucleus coil. The frequency gap between these two nuclei Larmor frequencies at 11.7 T is around 127 MHz (76-203 MHz). The wire-array coil consists of 5 variable-length brass wires connecting two PCBs with 5 metallization strpis (or patches) on each side of the wire (Figure 1, A). The patches serve as one plate of the structural capacitance that provides coil tuning capability. The opposing plates of the capacitors are formed by four PCBs fully metallized on one side placed above and below the PCBs with patches, thus forming an array of two parallel connected capacitances. The four outer boards can be moved relative to each other to change the overlap between the middle PCB with patches and outer PCBs, thus varying the total capacity. The variation in the capacity attached to the wires changes the wire array eigenmode frequency allowing the coil to be tuned to a particular Larmor frequency. The second coil is a butterfly loop coil7 tuned to 500 MHz (Figure 1, B). It employs two variable capacitors for tuning and matching. The coil is implemented as a double-sided PCB. Such coil configuration was chosen because the RF magnetic field it produces is predominantly directed orthogonally to the RF magnetic field produced by the X-nuclei coil which serves to reduce the mutual coil coupling. The radiofrequency coil design was simulated in the CST Microwave Studio 2017. Simulations were performed in the presence of the RF shield model and the phantom imitating average tissue properties of a small animal8. After determining the system parameters in simulation, a prototype of the multi-nuclei coil was assembled (Figure 1, C) and on-bench measurements were made. To prove that the prototype can be tuned to any frequency in the 76-203 MHz range, the coil resonant frequency (measured by Keysight PNA E8362C VNA as the coil S11 parameter minimum position) was recorded at different patch/plate overlap values. Next, to be sure that the X-nuclei and 1H coils were decoupled S12 parameter was measured at three different tunings of the X-nuclei coil (76, 125.7 and 203 MНz). Finally, the field distributions at the same frequencies were obtained experimentally.
Results
Simulation results and experimental measurements of the system tuning capability are presented in Figure 2, A. Tuning curves in Figure 2, B show that the proposed X-nuclei RF-coil can be tuned to any frequency in the target 76-203 MHz range with various fields of view. The anticipated coil decoupling due to RF fields orthogonality was confirmed by calculated and measured S12 parameters of the complete double-coil setup for three X-nuclei coil geometries corresponding to three different system tuning frequencies. S11 value at 1H frequency (Figure 3) was below -30 dB, and the S22 value at the X-nuclei frequencies was below -12 dB for all three tested frequencies. S12 value at 1H and X-nuclei frequencies in all three tuning configurations was below -30 dB. The magnetic field distribution for both coils was obtained in simulations and experimentally. Here we demonstrate the field components for the X-nuclei coil and the proton coil (Figure 4).
Discussion
The designed dual coil setup allows generating the excitation RF-field and acquisition of signals from protons as well as from several biologically important nuclei at 11.7 T. The latter include 2H (76.753 MHz), 13C (125.721 MHz), 23Na (132.256 MHz), 129Xe (138.302 MHz) and 31P (202.404 MHz). The S-parameter measurements have shown the butterfly coil to be uncoupled from the X-nuclei coil. This allows independent tuning of the two coils, and thus provides a convenient way to obtain both the reference anatomical 1H images and functional X-nucleus images on the seleсted frequency. Field distribution for both coils was shown to provide large enough FoV for whole-body small-animal imaging. However, the exact imaging volume has to be determined with animal scanning in an actual MRI scanner.
Conclusions
A novel RF-coil design was introduced allowing performing heteronuclear MR-imaging on several different X-nuclei using a single coil assembly. This prospectively removes the need to interfere with the animal, when acquiring multiple X-nuclei datasets, thus promising better precision and diversity of heteronuclear imaging.
Acknowledgements
This work was funded by the Russian Science Foundation (Project 19-75-10104).References
1. Robinson SP, Boogaart A van den, Maxwell RJ, Griffiths JR, Hamilton E, Waterton JC. 31P-magnetic resonance spectroscopy and 2H-magnetic resonance imaging studies of a panel of early-generation transplanted murine tumour models. Br J Cancer 1998; 77: 1752–1760.
2. Kurhanewicz J, Vigneron DB, Ardenkjaer-Larsen JH, Bankson JA, Brindle K, Cunningham CH et al. Hyperpolarized 13C MRI: Path to Clinical Translation in Oncology. Neoplasia 2019; 21: 1–16.
3. Madelin G, Regatte RR. Biomedical applications of sodium MRI in vivo. Journal of Magnetic Resonance Imaging 2013; 38: 511–529.
4. Mugler JP, Altes TA. Hyperpolarized 129Xe MRI of the human lung. Journal of Magnetic Resonance Imaging 2013; 37: 313–331.
5. Hurshkainen A, Nikulin A, Georget E, Larrat B, Berrahou D, Neves AL et al. A novel metamaterial-inspired RF-coil for preclinical dual-nuclei MRI. Scientific Reports 2018; 8: 9190.
6. Zubkov M, Hurshkainen AA, Brui EA, Glybovski SB, Gulyaev MV, Anisimov NV et al. Small-animal, whole-body imaging with metamaterial-inspired RF coil. NMR in Biomedicine 2018; 31: e3952.
7. Kumar A, Bottomley PA. Optimized quadrature surface coil designs. Magn Reson Mater Phy 2007; 21: 41.
8. Kraszewski A, Stuchly MA, Stuchly SS, Smith AM. In vivo and in vitro dielectric properties of animal tissues at radio frequencies. Bioelectromagnetics 1982; 3: 421–432.
Figures