4219
An open dataset of 126 human brain field maps1Dept.of Radiology, Medical Physics, Medical Center University of Freiburg, Faculty of Medicine, University of Freiburg, Freiburg, Germany, 2Max Planck Institute for Biological Cybernetics, Tuebingen, Germany, 3Department for Biomedical Magnetic Resonance, University of Tübingen, Tuebingen, Germany
Synopsis
126 human brain field maps from 18 normal subjects in 7 head positions each are published. The data may be of interest for shim coil designers as well as fro susceptibility mapping research.
Introduction
Shimming the human brain with customized coils has raised increasing interest in the past few years1-9. At the ISMRM 2019, three different research groups have simultaniously published their results7-9 of designing shim coil arrays using acquired field maps. In the studies, the design methods are quite similar and are based on the stream function method10 and the singular value decomposition. However, each group has used a different dataset of human brain field maps. This difference may lead to substantially different wire layouts of the designed coil arrays. Moreover, the use of their own test datasets for each of the studies may limit the generalization ability of the designed coil array. In this work, we make our dataset of field maps publicly available.. The published dataset can be used to design a shim coil array and test the validity of the existing coil arrays. Additionally the field maps may useful for developing and designing of the susceptibility mapping algorithms.Methods
The proposed method of measurement and postprocessing of human brain field maps is as follows. Anatomical images and B0 field maps of heads of 18 different healthy adult volunteers (age: 30.8 ± 3.97,gender: 14 males and 4 females) were measured on a 3T scanner (Siemens Healthcare, Erlangen, Germany) by a field mapping sequence based on a multi‐echo 3D gradient echo(GRE) sequence. The imaging parameters were TE = 3.84/6.7/10.56 ms, TR = 14 ms, flip angle = 8, field of view $$$(FOV) = (256 \times{256} \times{224}) mm_3$$$ , and $$$128 \times{128} \times{112}$$$ voxels. The measured maps have an isotropic resolution of 2 mm. The selected FOV was large enough to cover the whole brain (including: cerebellum, midbrain, andpons) of all subjects.In order to assess the impact of head motion on $$$B_0$$$ maps each subject was measured seven times in different head positions. The first field map was measured in a reference head position considered optimal by an experienced radiographer. The other six maps were measured after rotation by approximately $$$\pm{5}$$$ about $$$x, y,$$$ and $$$z$$$ axes, respectively. To this end, a 2D multislice echo-planar imaging (EPI) sequence was used to quantify the head movement using a prospective acquisition correction algorithm11 and the subjects were guided via the intercom system to comply with the requirements of the protocol.
The non‐brain areas of all field maps were removed using the BET (Brain Extraction Tool)12 based on the magnitude images from the scanned field maps at the second echo. The field maps were then unwrapped using the phase unwrapping algorithm in Robinson et al13 based on multi‐echo phase images. The norm of the three‐dimensional gradient of the phase of the unwrapped maps weighted by the magnitude images was used to detect outliers (such as noise pixels at the boundary and occasional residual phase wraps) and modify the usable brain mask. Disconnected regions were removed from the updated mask. No further topologic operations (such as erosion) were applied to the mask to maximally preserve reliably detected field inhomogeneities close to the surface of the brain. A weak 7‐voxel median filter constrained to the mask was applied to the unwrapped field maps to remove the residual outliers. Histograms of selected field maps (e.g. withthe highest standard deviations within the data set) were visually inspected to confirm the reliable mask generation and the absence of apparent outliers. All 126 final field maps have been published at DOi: 10.5281/zenodo.3530387 along with the postprocessing scripts.
Results and discussion
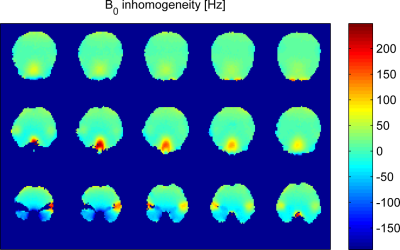
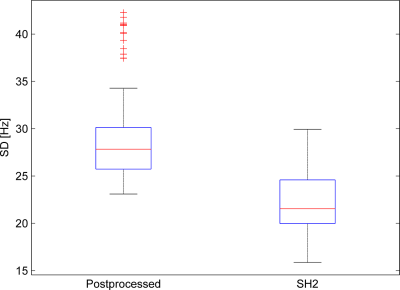
Figure 1 shows the resulting field inhomogeneities in some slices of a representive field map from the whole postprocessing procedure including the mask modification. Further field maps are being acquired and processed with a goal of increasing the number of participants to 30 and achieving a ginder balance.Figure 2 presents histograms of all the standard deviations (SD) for two different datasets. The first dataset consists of all the final 126 field maps after the postprocessing, as marked as 'Postprocessed' in Fig. 2. The second dataset is made of the resulting field maps from shimming the 126 field maps over the whole human brain using the second-order spherical harmonic (SH) shapes, as described as 'SH2' in Fig 2. As shown in the left histogram of Fig 2, the values of standard deviations have some extreme outliers. The outliers may be due to the large amount of anatomical diversity among the subjects. Moreover, after the second-order SH shimming, those extreme outliers disappear as illustrated in the right histogram of Fig 2.
Acknowledgements
This work was supported by German Research Foundation (DFG),Grant/Award Numbers: ZA 422/5‐1, ZA422/6‐1; Reinhart Koselleck Project,Grant/Award Numbers: DFG SCHE 658/12and SCHE 658/13‐1;References
1. Juchem et al. Magnetic field modeling with a set of individual localized coils. J Magn Reson. 2010;204:281-289.
2. Stockmann et al. A 32-Channel Combined RF and B0 Shim Array for 3T Brain Imaging. Magn Reson Med. 2016;75: 441-451.
3. Han et al. Integrated Parallel Reception, Excitation, and Shimming (iPRES). Magn Reson Med. 2013;70:241-247.
4. Truong et al. Integrated RF/shim coil array for parallel reception and localized B0 shimming in the human brain. NeuroImage. 2014;103:235-240.
5. Aghaeifar A, Zhou J, Heule R, Tabibian B, Schölkopf B, Jia F, Zaitsev M, Scheffler K. A 32‐channel multi‐coil setup optimized for human brain shimming at 9.4T. Magn Reson Med 2020;83:749–764.
6. Aghaeifar A, Mirkes C, Bause J, Steffen T, Avdievitch N, Henning A, Scheffler K. Dynamic B 0 shimming of the human brain at 9.4 T with a 16-channel multi-coil shim setup. Magn Reson Med 2018;80:1714–1725.
7. Jia F, Elshatlawy H, Aghaeifar A, et al. Design of a shim coil matched to the human brain anatomy. ISMRM 2019 Montreal p1475.
8. Arango N, Stockmann J, et al. Ultimate B0 Shim and the Design of Optimal Shim Bases. ISMRM 2019 Montreal p1462.
9. Meneses BP, Luong M, Amadon A. Optimized multi-coil array design for human brain shimming at Ultra-High Field. ISMRM 2019 Montreal p1477.
10. Peeren GN, Stream function approach for determining optimal surface currents, J Comput Phys. 2003;191(1):305‐321.
11. Thesen S, Heid O, Mueller E, et al. Prospective acquisitioncorrection for head motion with image‐based tracking for real‐time fMRI. Magn Reson Med. 2000;443:457-465.
12 Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17:143-155.
13. Robinson S, Schödl H, Trattnig S. A method for unwrapping highlywrapped multi‐echo phase images at very high field: umpire. Magn Reson Med. 2014;72:80-92.