4216
Overcome PNS for ultrasonic body gradients: concept
Edwin Versteeg1, Jeroen Siero1,2, and Dennis Klomp1
1Radiology, University Medical Center Utrecht, Utrecht, Netherlands, 2Spinoza Centre for Neuroimaging Amsterdam, Amsterdam, Netherlands
1Radiology, University Medical Center Utrecht, Utrecht, Netherlands, 2Spinoza Centre for Neuroimaging Amsterdam, Amsterdam, Netherlands
Synopsis
Ultrasonic MRI has been demonstrated successful for dedicated short head gradients running with slew rates over 5000T/m/s without experiencing painful peripheral nerve stimulation. However, this requires insert coils with complex cooling and high power connecters that limit its ease of use. More elegant would be to translate the technology to body gradients, yet operation of use will come to audible frequencies when avoiding PNS. Here we show a 20kHz setup that uses a 5 lobe polynomial rather than linear gradient in Z-direction combined with high channel SENSE unfolding that can reduce PNS 5-fold so to maintain ultrasonic.
Introduction
Previously, we introduced a method for silent imaging which incorporated a single-axis head gradient insert switching at 20 kHz for the sound dominant EPI train, which is an order of magnitude faster oscillation than traditional gradient systems can provide1. In this high frequency regime, PNS thresholds are limited by the absolute magnetic field generated by the oscillating gradient, which is acceptably small with short gradient inserts to prevent PNS. When translating the same ultrasonic method to the ~4-fold longer body gradients, PNS is expected to be too painful. However, the gradient could be split up in an array of shorter gradients, when receiver arrays can unfold the information. This way, the absolute field of oscillation maintains low as well as the associated PNS. Here we show simulations and a scaled setup to demonstrate the feasibility of using both high temporal oscillation as well as spatial oscillating fields in acquiring and reconstructing MRI.Methods
The scaled model composed of 4 sets of 8 windings each at an inter-set distance of 5cm wound around a 7cm diameter former (Fig 1). The current path of the odd sets was reversed to the even sets. The sets were connected in series and tuned with a capacitor to 20kHz and matched to 4ohm, which matches to the output impendence of the 5kW port of an audio amplifier (Powersoft, K20). A 32 channel high density receiver array was put on top of the gradient, which had 8 distinct elements aligned in Z-direction. While a dual layer gradient setup with shifted conductors is required for full encoding, we used a single port to demonstrate the encoding performance in two types of phantoms: one large phantom that encompassed the entire length of the sets of windings and two 4cm spheres placed in Z-direction. To setup the reconstruction pipeline, first a simulation was performed to demonstrate the ability to unfold the data and the consequence of using only one port and a 4-channel receive coil. Then the gradient strength was simulated using the power output of the amplifier, the losses in the coil, and the remaining currents to calculate the field. An FID with the oscillating gradient sets was obtained from the two spheres to verify the amplitude of spatial and temporal oscillation.Results
The reconstruction on the simulated data shows excellent unfolding capabilities even with a limited number of receive channels when using separated phantoms, and shows absence of encoding over the gradient transitions (Fig 2) for large phantoms. The peak to peak amplitude of the spatial and temporal fields was calculated to be 480mT/m with a spatial frequency of 1/10cm and the temporal frequency 19.5kHz when all power would be absorbed by the gradient. Based on the FID with the gradients switched on at 20, 40 and 80% of the maximum power, the maximum gradient can be estimated as 125mT/m (Fig 3) and the oscillation matches to 19.5kHz.Discussion and conclusion
We have shown a concept that may be the basis of ultrasonic body MRI by segmenting the gradient in Z-direction to mitigate PNS and driving the setup at the inaudible frequency of 20kHz. Simulations show that unfolding the data caused by gradient segmenting is feasible, yet a second shifted layer is required to reconstruct the image in transition areas where the gradient is low. While the scaled model is much smaller than a body gradient, it does facilitate experimental validation using simply available hardware.Acknowledgements
No acknowledgement found.References
1. Versteeg E, Klomp DWJ, Siero JCW. (2019), Supersonic imaging with a silent gradient axis driven at 20 kHz, #4586Figures
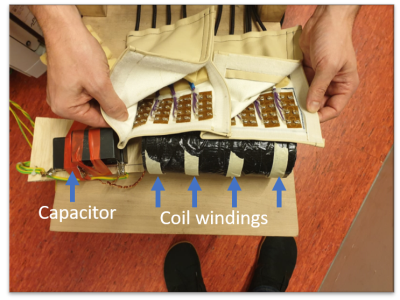
Figure
1
The constructed gradient with on top the 32-channel receive array
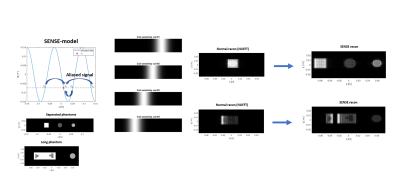
Figure 2 Simulation of the effect of using sinusoidal
gradient field. Note the SENSE model which uses a combination of the gradient
field and coil sensitivities
to unfold the data.
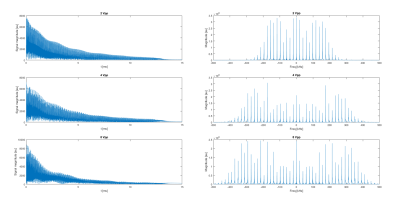
Figure 3 FID and magnitude spectrum of the FID used for
gradient estimation. Note the increased bandwidth of the spectrum at higher
gradient amplitude.