4198
Retrospective Analysis of Radio-frequency Safety of Orthopedic Passive Implantable Device
Kyoko Fujimoto1, An Smith1,2, Leonardo M Angelone1, Vikansha Dwivedi1, Sunder S Rajan1, Brent L Showalter1, and Nicole LL McMinn1
1Center for Devices and Radiological Health, US Food and Drug Administration, Silver Spring, MD, United States, 2University of Virginia, Charlottesville, VA, United States
1Center for Devices and Radiological Health, US Food and Drug Administration, Silver Spring, MD, United States, 2University of Virginia, Charlottesville, VA, United States
Synopsis
Implantable medical devices marketed in the US may include MRI Safety Information, to allow patients with such devices to safely undergo MR scans. However, some implantable devices are not evaluated for MR compatibility due to constraints of the testing cost and other resources. Not having MRI Safety Information in labeling limits patients’ access to MR scans. This study aimed to perform a retrospective analysis of MR safety testing data included in previously cleared 510(k) submissions for implantable orthopedic devices, with the goal to generate datasets that may help establish guidelines that potentially reduce the regulatory burden for future pre-market submissions.
INTRODUCTION
A Magnetic Resonance Imaging (MRI) scanner is considered a non-significant risk device if operated within specified parameters. Yet there are some safety concerns, including radio-frequency (RF) thermal injury, especially for patients with implantable devices. For simple, elongated devices, such as stents or rods, the RF induced heating is directly correlated with the length of the devices as previously shown with measurement data1; however, predicting heating of implantable devices with more intricate geometrical structures and sizes (e.g., complex orthopedic devices) can be challenging without appropriate testing.Medical device manufacturers perform Magnetic Resonance (MR) compatibility testing based on Standards2-6 and suggest either MR Safe, MR Conditional, or MR Unsafe labeling in premarket submissions to US Food and Drug Administration. The number of orthopedic devices with MR Conditional labeling has increased steadily over the past decade; however, testing is still limited by constraints related to cost and other resources including testing tools. As a result, some devices have a label of “MR Not Evaluated”, which limits patients’ access to MRI. This retrospective analysis of RF safety of implantable devices may help the manufacturers to estimate RF induced-heating of the devices. In this study we mined bench-testing measurements and computational modeling data from previously cleared 510(k) submissions in orthopedic passive implantable devices.
METHODS
Data mining was performed on 510(k) submissions for orthopedic devices cleared from January 2014 to June 30, 2019. The following information was gathered: locations of the device in clinical settings (“therapeutic location”), RF safety test type (ASTM phantom measurement, ASTM phantom modeling and anatomical human modeling), device dimensions, types of device components, and temperature increase results. All the temperature results due to RF heating were scaled to whole-body specific absorption rate (SAR) of 2 W/kg and were measured over a scan period of 15 minutes3.Based on the collected data, relationships between reported worst-case temperature increases and device dimensions were analyzed for the field strengths of 1.5T and 3.0T. Most of the submissions included multiple sizes of devices; thus, there were more data points than the number of the submissions. The analysis was focused on submissions that included both measurement and computational modeling data with the ASTM phantom.
RESULTS
A total of 214 submissions were identified with MR Conditional labeling. The number of submissions cleared with MR Conditional labeling has been increasing over six years (Fig.1). The number of submissions in 2018 was almost equal to the number of submissions from January to June in 2019. The submissions were then categorized in 12 therapeutic locations (Fig.2). Some devices targeted multiple therapeutic locations.The spine submissions were further analyzed as it was most common (28%) in the orthopedic devices. There were 23 submissions which included testing of both measurements and modeling using the ASTM phantom out of the spine submissions. Five submissions included the results from modeling utilizing an anatomical human model to demonstrate potential induced heating at the therapeutic location. There were up to 88 total data points from this subset of data: 40 for measurement, 27 for ASTM phantom modeling, and 21 for anatomical human modeling. The reported uncertainty was around 25%. The device components included rods, screws, plates, or cages, which were made of titanium, titanium-alloy and/or polymers.
Reported temperature increases at 1.5T and 3.0T are summarized in Figure 3. Average increases in temperature were 7.5°C ± 4.4°C at 1.5T and 5.3°C ± 3.2°C at 3.0T for ASTM phantom measurement, 6.9°C ± 4.1°C at 1.5T and 4.9°C ± 2.4°C at 3.0T for ASTM phantom modeling, and 3.3°C ± 2.2°C at 1.5T and 3.6°C ± 1.9°C at 3.0T for anatomical human modeling.
Device lengths and temperature increases from measurement results were compared (Fig.4). The distribution suggests that the relationship between temperature increase and device length depends on the field strengths.
DISCUSSION
Within data of submissions for spine devices, ASTM phantom measurement results reported higher temperature increases at both field strengths compared to that of computational modeling with ASTM phantom or anatomical human models. However, this may not be the case for devices which are implanted at multiple therapeutic locations such as small bone implantable devices. For such devices, modeling results with anatomical human models may reveal higher temperature increase than those of measurement with ASTM.Variability in temperature data may have been impacted by the variability in device dimensions other than length. Furthermore, challenges faced in the development of this database include a lack of information regarding certain detailed parameters. Future studies include devices for different therapeutic locations, detailed dimensions including width and diameter and, the orientation of the device on the anatomy of the body as they can also contribute to RF-induced heating in addition to lengths.
CONCLUSION
MR RF heating retrospective analysis was performed on 214 cleared 510(k) orthopedic submissions with a focus on spinal implantable devices. Comparisons of measurement and modeling data revealed that ASTM phantom measurement data estimates higher temperature increase for spine implantable devices. More rigorous analyses for other therapeutic locations may help the orthopedic device manufacturers for MR conditional labeling.Acknowledgements
DISCLAIMER: The mention of commercial products, their sources, or their use in connection with material reported herein is not to be construed as either an actual or implied endorsement of such products by the Department of Health and Human Services.References
- Song T, Xu Z, Iacono MI, et al. Retrospective analysis of RF heating measurements of passive medical implants. Magn. Reson. Med. 2018;80(6):2726-2730.
- ASTM F2053 – 13 Standard Practice for Marking Medical Devices and Other Items for Safety in the Magnetic Resonance Environment. ASTM International, West Conshohocken, PA, 2013, DOI: 10.1520/F2503-13, www.astm.org
- ASTM F2182 – 11a Standard Test Method for Measurement of Radio Frequency Induced Heating On or Near Passive Implants During Magnetic Resonance Imaging. ASTM International, West Conshohocken, PA, 2011, DOI: 10.1520/F2182-11A, www.astm.org
- ASTM F2052 – 14 Standard Test Method for Measurement of Magnetically Induced Displacement Force on Medical Devices in the Magnetic Resonance Environment. ASTM International, West Conshohocken, PA, 2014, DOI: 10.1520/F2052-14, www.astm.org
- ASTM F2119 – 07 Standard Test Method for Evaluation of MR Image Artifacts from Passive Implants. ASTM International, West Conshohocken, PA, 2013, DOI: 10.1520/F2119-07, www.astm.org
- ASTM F2213 – 17 Standard Test Method for Measurement of Magnetically Induced Torque on Medical Devices in the Magnetic Resonance Environment. ASTM International, West Conshohocken, PA, 2013, DOI: 10.1520/F2213-17, www.astm.org
Figures
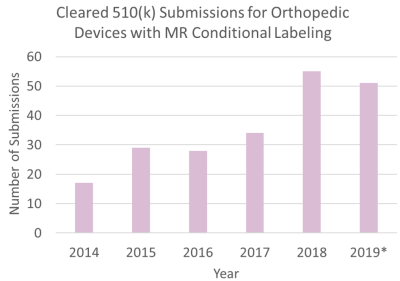
Figure 1: The total number of the cleared 510(k) submissions for
orthopedic implantable devices with MR Conditional labeling is shown for each year.
There were 214 submissions from January 1st, 2014 to June 30th, 2019. The value for 2019 is
partial, as indicated with an asterisk.
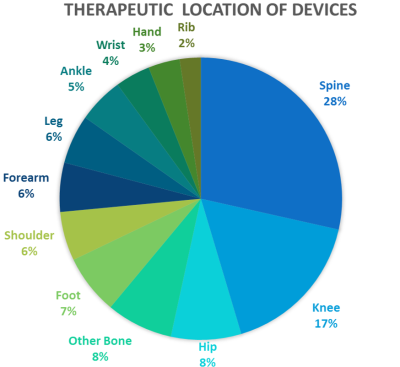
Figure 2: Therapeutic locations were identified in the 214
submissions. Some devices had more than one target therapeutic location.
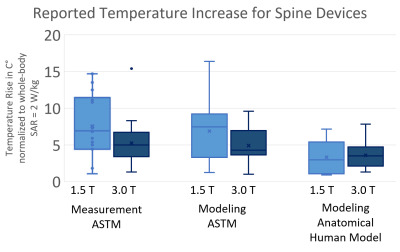
Figure 3: Reported temperature increases for spine devices were
analyzed among ASTM phantom measurement, ASTM phantom modeling and anatomical
human modeling for both 1.5T and 3.0T.
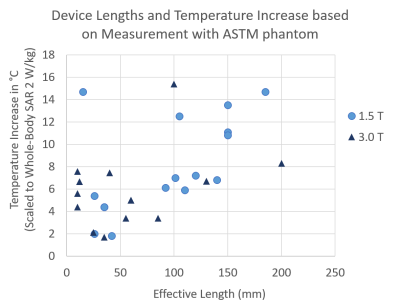
Figure 4: Device lengths and temperature increase results from
measurement with ASTM phantom were compared.