4193
Tier based formalism for RF safety assessment of custom-built RF coils providing flexibility in the tradeoff between effort and overestimation1Center for Image Sciences, Radiology, University Medical Center Utrecht, Utrecht, Netherlands, 2Center for Image Sciences, Radiotherapy, University Medical Center Utrecht, Utrecht, Netherlands, 3Biomedical Engineering, Medical Image Analysis, Eindhoven University of Technology, Eindhoven, Netherlands
Synopsis
We propose an RF safety assessment formalism that uses four tier levels to quantify the accuracy of SAR modeling. Each subsequent tier level consists of more modeling and validation efforts while reducing the overestimation of peak SAR levels. The lowest tier level requires no simulations or measurements whereas the highest tier level includes B1+ and thermometry-based validations. The formalism defines a safety factor to account for deviations between the simulation model and the subject adding errors with the sum-of-squares method. A tier 3 validation and error propagation procedure was conducted for a 7T prostate array.
Introduction
To ensure the safe use of custom-built RF coils in MRI, normally electromagnetic (EM) simulations are performed to calculate peak local SAR levels. These simulations are used to derive safe average input power limits based on the peak local SAR limits from the IEC1. The peak (local) SAR level determined by simulations will deviate from the actual peak SAR level in the patient. Therefore, a safety factor is typically applied to account for these potential deviations. Three potential sources of deviation are identified: inter-subject variability of SAR, inaccuracies in the coil model and inaccuracies in average power monitoring on the scanner2. The assessment of these three sources of uncertainty is part of any RF safety assessment procedure2–7. Presented procedures consist of one approach that aims for the highest accuracy with typically extensive modeling and validation efforts8. We propose an alternative RF safety assessment formalism that provides flexibility to trade low overestimation and extensive efforts for larger overestimations and more time-efficient procedures. Our method focuses specifically on calculating the modeling inaccuracy, which we define as the potential difference between the simulation model and the subject. Similar to the formalism for RF safety assessment of active implanted medical devices flexibility is provided by four tier levels9. Each subsequent tier level consists of more extensive modeling and validation efforts while reducing the overestimation of peak SAR levels by arriving at a lower modeling inaccuracy.Methods
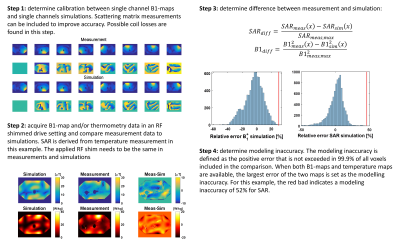
The tier levels are defined in table 1. Tier 0 assumes all power is deposited in 10g of tissue10. Tier 1 performs numerical simulations without validation and compensates by a large overestimation of the modeling inaccuracy. Tier 2 performs simulations and validations based on B1+ mapping or MR thermometry. Modeling inaccuracy is determined by phantom validations. Tier 3 performs validations by B1+ mapping and MR thermometry. Now coil losses are no longer neglected resulting in minimal SAR overestimation.To provide an example of how to calculate the modeling inaccuracy, the full validation procedure was followed up to tier level 3 for a 7T fractionated dipole array11. All simulations were done in Sim4Life (Zurich Med Tech, Zurich, Switzerland). All measurements were done on a polyviniylpyrrolidone12 (PVP) phantom (εr=37, σ=0.4 S/m). Figure 1 shows the proposed workflow to arrive at validation tier level 3. First, the calibration between measured and simulated single-channel B1+-maps was found. In this case, a complex scaling factor was found for every channel of the transmit array. After these scaling factors were known, B1+ measurements and temperature measurements were done with the full array and a specific RF shim. These measurements were compared to simulations where the same RF shim is used. Finally, to determine the modeling inaccuracy, the difference between the spatial B1+-maps and the SAR maps was calculated. The modeling inaccuracy is then defined as the positive error that is not exceeded in 99.9% of all voxels. When both B1+-maps and temperature maps are available, the largest error of the two maps is set as the modeling inaccuracy.
After the modeling inaccuracy was quantified with the tier level method and the inter-subject variation and power monitoring error were found from literature, the different sources of inaccuracy were combined into a total safety factor which was used to arrive at a corrected peak SAR level.
$$SAR_{corr} = SAR_{sim} * SF$$ [1]
Here SARcorr is the corrected peak SAR level, SARsim is the peak SAR level that resulted from simulations and SF is the safety factor (SF>1). The safety factor follows from the relative peak SAR uncertainty, where ΔSARtotal is the total uncertainty on the simulated peak SAR level SARsim:
$$SF = 1+\frac{ΔSAR_{total}}{SAR_{sim}}$$ [2]
ΔSARtotal follows from the individual sources of uncertainty ΔSARint.subj.var, ΔSARmodel.inaccuracy and ΔPinput .
$$(\frac{ΔSAR_{total}}{SAR})^2=(\frac{ΔP_{input}}{P_{input}})^2+(\frac{ΔSAR_{int.subj.var}}{SAR})^2+(\frac{ΔSAR_{model.inaccuracy}}{SAR})^2$$ [3]
Because all these sources of error are independent of each other, error propagation was calculated using the sum-of-squares method.
Results
For prostate imaging, Meliado et al found an average peak SAR of 2.4 W/kg for 8x1 W input power and an inter-subject variation error of 80%6. The power measurement error is 10% based on directional coupler specifications. Figure 1 shows the results of the validation, in which the thermometry shows a modeling inaccuracy of 52%. The total error adds up to 98%, leading to a safety factor of 1.98 (table 2) for random RF shim settings.Conclusion
A formalism is presented for RF safety assessment of multi-transmit coil arrays, which classifies the level of simulation and validation effort in a tier system. The largest tier level provides minimum overestimation at the expense of considerable modeling and validation efforts and vice versa. Modeling inaccuracy is determined by a statistical approach based on differences between simulated and measured B1+-maps/temperature maps. To address total peak local SAR uncertainty, predicted peak local SAR levels are multiplied by a safety factor which is obtained by adding individual sources of uncertainty in a sum-of-squares way. For the investigated dipole antenna array the resulting per channel power limit is 4.2 W.Acknowledgements
We would like to acknowledge de Dutch Technology Foundation TTW, grant number 3507 for providing the funding for this project.References
1. IEC. Medical electrical equipment. Part 2-33: particular requirements for the safety of magnetic resonance equipment for medical diagnosis. IEC 60601-2-33. 2015.
2. Boulant N, Gras V, Amadon A, Luong M, Ferrand G, Vignaud A. Workflow proposal for defining SAR safety margins in parallel transmission. In: Proceedings of the 26th Annual Meeting of ISMRM, Paris, France. ; 2018:0295.
3. Ferrand G, Luong M, Amadon A, Boulant N. Mathematical Tools to Define SAR Margins for Phased Array Coil In-Vivo Aplications Given E-field Uncertainties. In: Proc. Intl. Soc. Mag. Reson. Med. 23 Toronto. ; 2015:1862.
4. De Greef M, Ipek O, Raaijmakers AJE, Crezee J, Van Den Berg CAT. Specific absorption rate intersubject variability in 7T parallel transmit MRI of the head. Magn Reson Med. 2013;69(5):1476-1485. doi:10.1002/mrm.24378
5. Ipek Ö, Raaijmakers AJ, Lagendijk JJ, Luijten PR, Van Den Berg CAT. Intersubject local SAR variation for 7T prostate MR imaging with an eight-channel single-side adapted dipole antenna array. Magn Reson Med. 2014;71(4):1559-1567. doi:10.1002/mrm.24794
6. Meliadò EF, van den Berg CAT, Luijten PR, Raaijmakers AJE. Intersubject specific absorption rate variability analysis through construction of 23 realistic body models for prostate imaging at 7T. Magn Reson Med. 2018;(April 2018):2106-2119. doi:10.1002/mrm.27518
7. Le Garrec M, Gras V, Hang MF, Ferrand G, Luong M, Boulant N. Probabilistic analysis of the specific absorption rate intersubject variability safety factor in parallel transmission MRI. Magn Reson Med. 2017. doi:10.1002/mrm.26468
8. Hoffmann J, Henning A, Giapitzakis IA, et al. Safety testing and operational procedures for self-developed radiofrequency coils. NMR Biomed. 2016. doi:10.1002/nbm.3290
9. 10974:2012(en) I. Assessment of the safety of magnetic resonance imaging for patients with an active implantable medical device. https://www.iso.org/standard/46462.html. Published 2012.
10. Vignaud A, Mauconduit F, Gras V, et al. Fast and unconditionally safe in vivo MR head protocol for home-made coil prototype assessment at 7T. In: Proc. 36th ESMRMB, Rotterdam. ; 2019:1289.
11. Raaijmakers AJE, Italiaander M, Voogt IJ, et al. The fractionated dipole antenna: A new antenna for body imaging at 7 Tesla. Magn Reson Med. 2016;75(3):1366-1374. doi:10.1002/mrm.25596
12. Ianniello C, de Zwart JA, Duan Q, et al. Synthesized tissue-equivalent dielectric phantoms using salt and polyvinylpyrrolidone solutions. Magn Reson Med. 2018;80(1):413-419. doi:10.1002/mrm.27005
Figures