4189
A method for MR system SAR control
Xin Chen1, Sadanori Tomiha2, Shinji Mitsui2, Yoshinori Hamamura1, and Michael Steckner1
1Canon Medical Research USA, Inc., Mayfield Village, OH, United States, 2Canon Medical Systems Corporation, Otawara-shi, Japan
1Canon Medical Research USA, Inc., Mayfield Village, OH, United States, 2Canon Medical Systems Corporation, Otawara-shi, Japan
Synopsis
This work demonstrates a SAR calculation method that manages the relationship between power deposition and patient body habitus.
INTRODUCTION
RF power deposition is a major safety concern for MR scans. MR scanner reported SAR must stay below certain limits to prevent adverse effects. Independently measured SAR showed big differences from scanner reported SAR.1 MR scanner’s SAR control needs to conservatively cover the entire patient population and all scan conditions to a degree determined by risk assessment, hence resulting in significant safety margins for the average patient. Future development of thermal dose-based safety control2 also requires accurate assessment of SAR. In this study we propose a whole body SAR calculation method that manages the relationship between power deposition and patient body habitus.METHODS
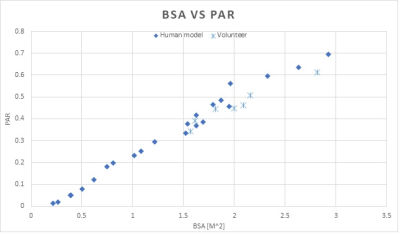
Numerical simulations are performed with Sim4Life (v4.4, ZMT). A standard birdcage whole body Tx coil loaded with different human models3-6 is modeled. We define Power Absorption Ratio (PAR) asPAR = Power loss in human model/Total net input power of the Tx coil [1]
where net input power is forward power minus reflected power. PAR is calculated for each human model and plotted vs certain metrics of body size (e.g. Body Surface Area, BSA) with high statistical correlation (Figure 1). In addition, PAR is measured with seven volunteers on a clinical 3T scanner. RF power loss in each volunteer is measured with pulse energy method7, and then divided by the net power delivered to the Tx coil (reported by the RF amp) to calculate PAR.
RESULTS
Figure 1 shows that both modeled and measured PAR show strong correlation with BSA, as larger BSA leads to higher PAR, and vice versa. Note that there are no exact matches between human models and volunteers, but modeling and experiment data show good overlapping as well as same trend.DISCUSSION
The key of MR scanner SAR control is assessing RF power loss in patient (which cannot be directly measured in-vivo) under a large variety of scan conditions. In other words, the unknown factor is PAR as defined in Equation 1, as the total input power to the Tx coil is known from RF amplifier and other factors (with measurement error considered). Since a human body is roughly elliptical/cylindrical shaped, a larger body with larger frontal projection area or body surface area likely has larger eddy current loops when exposed to time-varying B1 field. This then means more power is deposited into the body. Previously published work showed correlation between peak local SAR and anatomical properties.8 The presented modeling and experiment results show that the portion of Tx coil input power that is absorbed by human body is strongly correlated with body habitus. While we expect other anatomical properties (e.g. frontal projection area) that correlates with eddy current loop size may also show strong correlation with PAR, BSA can be easily calculated with patient height and weight9. A curve fitting (e.g. linear regression) may be applied to PAR data shown in Figure 1, and further statistical analysis can be used to generate safety margin for specific risk factor.CONCLUSION
The portion of power delivered to Tx coil that is absorbed by human body is shown to have strong correlation with body surface area. A SAR control method is proposed based on this finding.Acknowledgements
References
- El-Sharkawy AM, Qian D, Bottomley PA, and Edelstein WA. A multichannel, real-time MRI RF power monitor for independent SAR determination. Med. Phys. 2012; 39: 2334–2341.
- Murbach M, Neufeld E, Capstick M, et al. Thermal Tissue Damage Model Analyzed for Different Whole-Body SAR and Scan Durations for Standard MR Body Coils. Mag. Reson. Med. 2014;71: 421-431.
- Christ A, Kainz W, Hahn EG, et al. The Virtual Family—development of surface-based anatomical models of two adults and two children for dosimetric simulations. Phys Med Biol. 2010;55:N23.
- Gosselin M-C, Neufeld E, Moser H, et al. Development of a new generation of high-resolution anatomical models for medical device evaluation: The virtual population 3.0. Phys Med Biol. 2014;59:5287.
- Petoussi-Henss N, Zankl M, Fill U, Regulla D. The GSF family of voxel phantoms. Phys Med Biol. 2002;47:89.
- Menzel HG, Clement C, DeLuca P. ICRP Publication 110. Realistic reference phantoms: An ICRP/ICRU joint effort. A report of adult reference computational phantoms. Annals of the ICRP. 2009;39:1.
- NEMA Standards Publication MS 8-2016: Characterization of the Specific Absorption Rate (SAR) for Magnetic Resonance Imaging Systems.
- Murbach M, Neufeld E, Kainz W, et al. Whole-Body and Local RF Absorption in Human Models as a Function of Anatomy and Position within 1.5T MR Body Coil. Mag. Reson. Med. 2014;71: 839-845.
- Haycock GB, Schwartz GJ, and Wisotsky DH. Geometric method for measuring body surface area: A height-weight formula validated in infants, children, and adults. The Journal of Pediatrics 1978; 93: 62-66.