4185
Heating risk by radiofrequency and switching gradient fields in the presence of metallic implants
Valeria Clementi1, Umberto Zanovello2, Alessandro Arduino2, Cristina Ancarani1, Barbara Bordini1, Oriano Bottauscio2, Mario Chiampi2, Luca Zilberti2, and Fabio Baruffaldi1
1Medical Technology Laboratory, IRCCS Istituto Ortopedico Rizzoli, Bologna, Italy, 2Istituto Nazionale di Ricerca Metrologica (INRIM), Torino, Italy
1Medical Technology Laboratory, IRCCS Istituto Ortopedico Rizzoli, Bologna, Italy, 2Istituto Nazionale di Ricerca Metrologica (INRIM), Torino, Italy
Synopsis
A growing number of patients with metallic implants are subjected to MRI exams, but a patient-specific heating risk assessment is not easily available. After a frequency analysis of databases collecting prosthetic patients data, a heating risk assessment, based on existing standards for RF fields and recent scientific studies for switching gradient coil fields, is performed. Results show that the whole body SAR estimation provided by the MRI scanner is not a self-reliant safety index for patients with metallic implants and suggest the need for a general procedure involving both RF and gradient coil fields induced heating.
INTRODUCTION
The number of patients with a metallic implant to whom an MRI exam1 is prescribed is becoming larger and larger. However, radiologists’ need of patient-specific heating risk assessment is often not supported by sufficient or easily usable information. It is widely known that, in the presence of a metallic implant, the RF field may lead to local enhancement of the electric field causing power deposition directly inside the biological tissues2,3. On the contrary, the indirect thermal effect of the switching gradient coil (GC) field, that may heat the implant by inducing eddy currents inside its metallic parts, has received attention only in recent scientific literature4,5,6. In view of a standardization process, we propose an approach based on already existing standards for RF fields7 and available scientific studies for GC fields providing useful elements to guide the identification of the heating risk conditions.METHODS
Data on the population with prosthetic implants subjected to MRI exams have been extracted by the match between hip, knee and shoulder arthroplasty performed during year 2013, from the Register of the Orthopaedic Prosthetic Implants (RIPO) report (2016) of the Emilia-Romagna region (Italy), and MRI exams executed during the following three years (2013-2016), from the regional databases of clinical services provided to the population by the National Healthcare System. On the one hand, RF field effects have been studied with the Sim4Life software by computing the SAR distribution within an ASTM-like phantom containing the implant and radiated by a birdcage body-coil. For different MR pulse sequences, the volume and maximum local SAR averaged over 10 g masses, have been averaged over 6 minutes7. Moreover, the local SAR has been averaged over the sequence duration for comparisons. As regards the thermal effects of the GC fields, which intrinsically involves many parameters, the eddy currents induced by the switching GC within the metallic implant have been computed numerically by neglecting the skin effect (which appears at higher frequencies) and assuming a homogeneous magnetic field distribution; i.e. the magnetic field generated by each GC in the barycentre of the prosthesis. In the procedure, the actual waveforms of the currents running in the GC have been decomposed by Fourier transform. The computed eddy currents have been used to estimate the deposed power, which has been then employed as the forcing term of the bio-heat equation8 in order to estimate the maximum temperature increase after the sequence duration (ϑ) and at steady-state (ϑ∞). To this end, the prosthesis has been assumed to be completely surrounded by muscle. It is worth saying that higher values of temperature increase are likely to occur if another material with lower blood perfusion or thermal conductivity, like bone or a phantom gel, is simulated.RESULTS
Based on the database frequency analysis (Fig. 1), the described methods have been applied for studying the RF and GC effects on a hip, knee, or shoulder implant carrier for imaging of the head and pelvis (Fig. 2). Despite their high rate of occurrence, musculoskeletal and spine MRI exams have been only partially included in the pelvis imaging, since data does not allow to identify the longitudinal position of the patient within the coils for these exams. Computations have been performed for the pulse sequences collected in Fig. 3, assuming a closed bore 1.5 T scanner. Head and pelvis imaging results are presented in Fig. 4 and 5, where only the implants significantly exposed to RF or GC fields are reported. All the studied sequences comply with the limits defined on global and local SAR7 in absence of the implants (2 W/kg and 10 W/kg, respectively).DISCUSSION
During head imaging, only the shoulder prosthesis is exposed to both RF and GC fields. Differently, all implants are involved when the pelvis represents the target region. The RF local hotspots are enhanced when the implant is near the scanned region. The opposite situation occurs for the GC, because of the increase of the field values moving away from the coil isocenter. For RF, the worst condition is represented by the True FISP, mainly due to its short TR, whereas the EPI sequence generally leads to the highest temperature increase for GC interactions, due to its long gradient echo train. In all cases, the SARVol,6min (a parameter evaluated in real time by MRI scanners, which usually does not consider the presence of the implants) is lower than the limits7 even in those scenarios with higher temperature increase and local SAR.CONCLUSION
The estimation, provided by each MRI scanner, of the whole body SAR which is likely to be produced during the execution of a specific sequence, is doubtless a fundamental information. However, it is not a self-reliant parameter to define the MR exam safety when patients with metallic implants are involved. In those scenarios, such a parameter can be substantially misleading, not providing any detail about potentially dangerous localized SAR peaks and temperature increase due to GC. The results of this study suggest the need for standards and general procedures able to identify the risks associated with MRI exams of implant carriers involving both RF and GC fields.Acknowledgements
The results here presented have been developed in the framework of the 17IND01 MIMAS Project. This project has received funding from the EMPIR Programme, co-financed by the Participating States and from the European Union’s Horizon 2020 Research and Innovation Programme.References
- OECD/EU. Health at a Glance: Europe 2016 – State of Health in the EU Cycle. Paris, France: OECD Publishing; 2016.
- Ho HS. Safety of metallic implants in magnetic resonance imaging. J Magn Reson Im. 2001;14:472-477.
- Nadja A. 3-T MRI implant safety: heat induction with new dual-channel radiofrequency transmission technology. Eur Radiol Exp. 2018;2:7.
- Graf H, Steidle G, Schick F. Heating of Metallic Implants and Instruments Induced by Gradient Switching in a 1.5-Tesla Whole-Body Unit. J Magn Reson Im. 2007;26:1328–1333.
- Zilberti L, Bottauscio O, Chiampi M, et al. Numerical Prediction of Temperature Elevation Induced around Metallic Hip Prostheses by Traditional, Split, and Uniplanar Gradient Coils. Magn Reson Med. 2015; 74:272–279.
- Zilberti L, Arduino A, Bottauscio O, et al. The Underestimated Role of Gradient Coils in MRI Safety. Magn Reson Med. 2017, 77:13-15.
- IEC 60601-2-33: Particular requirements for the safety of magnetic resonance equipment for medical diagnosis.
- Arduino A., Bottauscio O., Chiampi M, and Zilberti L., “Douglas–Gunn Method Applied to Dosimetric Assessment in Magnetic Resonance Imaging”, IEEE Trans. Magn. 2017, 53:5000204.
Figures
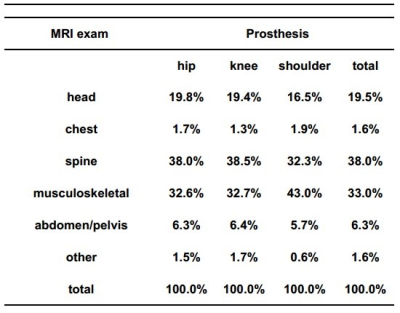
Figure 1: MRI exams on prosthetic patients during the three years (2013-2016)
after the arthroplasty surgery in 2013.
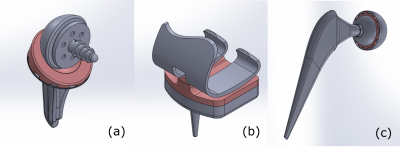
Figure 2: CAD models
of the implants studied. Shoulder implant (a), knee implant (b) and hip implant
(c).

Figure 3:
Considered sequences and corresponding durations.
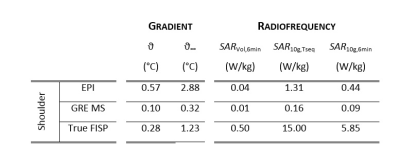
Figure 4: RF and GC
results for head imaging. ϑ, ϑ∞ are the maximum temperature increase
within the implants after the sequence execution and at steady-state,
respectively. SARVol,6min
and SAR10g,6min are the
volume SAR and maximum local SAR, respectively, averaged over 6 minutes. SAR10g,Tseq is the maximum local
SAR averaged on the sequence duration. Results are collected only for the
shoulder implant, because knee and hip implants are not significantly exposed
to RF or GC fields.
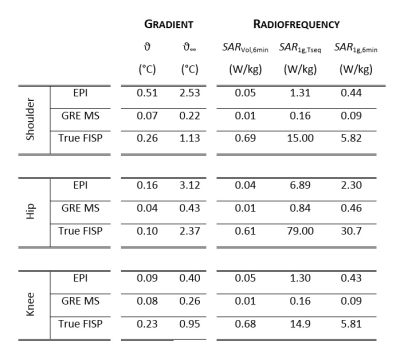
Figure 5:
RF and GC results for pelvis imaging. ϑ, ϑ∞ are the maximum
temperature increase within the implants after the sequence execution and at
steady-state, respectively. SARVol,6min
and SAR10g,6min are the
volume SAR and maximum local SAR, respectively, averaged over 6 minutes. SAR10g,Tseq is the maximum
local SAR averaged on the sequence duration.