4176
Interdependency of SAR amplification on external trajectory and internal geometry of implanted leads during MRI at 3T1Department of Radiology, Northwestern University, Chicago, IL, United States
Synopsis
One of the most crucial MRI safety concerns for patients with implanted leads, such as deep brain stimulation or spinal cord simulation implants, is to predict the worst-case RF heating. Simulations are increasingly used to search for lead trajectories that generate maximum SAR amplification in the tissue, however, due to limited computing resources such models dramatically simplify internal structure of the leads. Here we show that both the internal geometry and trajectory of implanted leads need to be accounted for reliable SAR prediction. Contrary to the general belief, leads with tightly-coiled internal wires do not always generate less RF heating.
Introduction
It is well known that the trajectory of an implanted lead significantly affects its RF heating during MRI [1-4]. As the FDA now promotes use of clinically relevant models to assess MRI safety [5], researchers have increasingly used simulations to predict worst-case RF heating scenarios in patients with implanted leads, either to design new MRI hardware [6-17], assess safe imaging parameters [18, 19], or design new MR-conditional leads [20, 21]. These simulations however, dramatically simplify internal geometry of the leads. Notably, they typically use straight wires to model the tightly-coiled helical structure interconnections that are now present in most MR-conditional leads. Here we show that omitting such details can significantly shift the prediction of lead trajectories that generate worst-case RF heating during MRI at 3T. Furthermore, our simulations show that contrary to the status-quo belief, leads with coiled wires do not always generate less RF heating.Methods
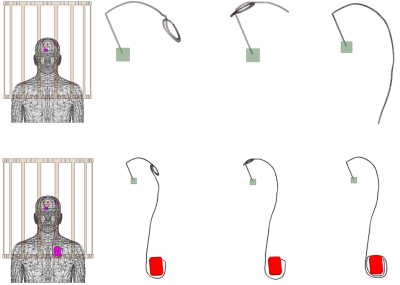
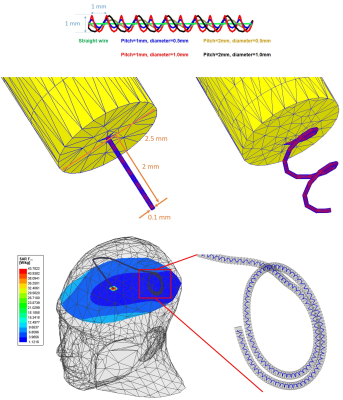
We developed models of a deep brain stimulation (DBS) device with two configurations: (a) a lead-only system, and (b) a fully-implanted system including an extension and an implanted pulse generator (IPG) inside an MRI body coil tuned at 127 MHz. DBS surgery is typically performed at two stages. First, leads are implanted in specific brain nuclei and the extracranial portion of the lead is tucked under skin for later connection to the IPG. MRI at this stage is useful for electrode localization. In the next stage, leads are connected to the IPG via subcutaneous extensions. Most MRI exams in DBS patients are performed on fully implanted systems. The full model consisted of a 40 cm lead, connected and a 56 cm extension, and a pulse generator implanted in the left pectoral region. The lead and extension consisted of a metallic core of 100 mm diameter surrounded by a 2.5 mm insulation and a 2 mm exposed tip. We simulated three DBS lead/extension trajectories typically observed in our patients: (1) the extracranial portion of the lead being looped at the surgical burr hole (2) the lead being looped on the temporal bone, and (3) no loop in the trajectory of the lead, extension looped around the IPG. For each trajectory, we investigated five different internal lead/extension geometries, including (a) a straight core, (b) helical core with the pitch of 1 mm and dimeter of 0.5 mm, (c) helical core with pitch of 1 mm and diameter of 1 mm, (d) helical core with pitch of 2 mm and diameter of 0.5 mm and (e) helical core with pitch of 2 mm and diameter 1 mm (see Figure 2). The DBS device was positioned in a homogenous body model with electrical conductivity σ = 0.5 S/m and relative permittivity εr = 69 representing for average human tissue. The body model was positioned inside a body coil (length = 62 cm, diameter = 61cm) tuned at 127 MHz with the head at the iso-center. Simulations were implemented in ANSYS Electronics Desktop and the maximum 1g-averaged SAR (MaxSAR1g) in a 2cm × 2cm × 2cm cubic area around the tip of the DBS leads during MRI at 3T (127 MHz).Results
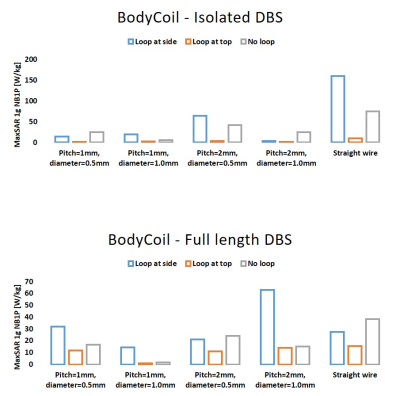
Figure 3 shows the MaxSAR1g calculated for all simulations of isolated DBS lead and fully implanted DBS systems. We report several interesting observations: First, the SAR around tips of the lead in a lead-only system was significantly larger than the SAR at tips of leads in a fully implanted system, a phenomenon that we have also observed in our RF heating experiments with commercial DBS devices; second, although having helical core is generally thought to reduce RF heating of an implanted lead during MRI by making it less prone to MRI-induced RF currents [22, 23], this may not be true at 3T for certain lead trajectories. Finally and most importantly, for both isolated and fully-implanted systems and for all internal lead structures, RF heating was highly sensitive to the trajectory of the lead. This is in agreement with previous reports on lead-only DBS systems [1-3]. Notably, looping a DBS lead at the surgical burr hole significantly recues the SAR with the effect being more prominent for lead-only systems. The result was constant over different lead trajectories and internal core geometries.Conclusion
Numerical simulations are increasingly used to inform worst-case scenarios in the context of MRI RF safety. Due to limited computing resources, such models usually simplify internal geometry of the implant. This can have significant ramifications in predicting RF heating around implanted leads which usually consist of multiple tightly-wound helical wires. Our results show that for a reliable prediction of worst-case SAR scenarios, both internal geometry of the lead and its trajectory need to be accounted for in modeling.Acknowledgements
This work has been supported by NIH grants R00EB021320, R03EB025344, and R03EB024705.References
[1] L. Golestanirad et al., "RF-induced heating in tissue near bilateral DBS implants during MRI at 1.5 T and 3T: The role of surgical lead management," NeuroImage, vol. 184, pp. 566-576, 2019.
[2] L. Golestanirad et al., "Variation of RF heating around deep brain stimulation leads during 3.0 T MRI in fourteen patient-derived realistic lead models: The role of extracranial lead management," Proc. Intl. Soc. Mag. Reson. Med. 25 2017.
[3] L. Golestanirad, L. M. Angelone, M. I. Iacono, H. Katnani, L. L. Wald, and G. Bonmassar, "Local SAR near deep brain stimulation (DBS) electrodes at 64 MHz and 127 MHz: A simulation study of the effect of extracranial loops " Magnetic Resonance in Medicine vol. 88, no. 4, pp. 1558-1565, 2016. [4] E. Mattei et al., "Complexity of MRI induced heating on metallic leads: experimental measurements of 374 configurations," Biomedical engineering online, vol. 7, no. 1, p. 11, 2008.
[5] "U. S. Food and Drug Administration, "Reporting of Computational Modeling Studies in Medical Device Submissions -Guidance for Industry and Food and Drug Administration Staff", http://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/UCM381813.pdf," 2015.
[6] C. McElcheran et al., "Numerical Simulations of Realistic Lead Trajectories and an Experimental Verification Support the Efficacy of Parallel Radiofrequency Transmission to Reduce Heating of Deep Brain Stimulation Implants during MRI," Nature Scientific Reports vol. 9, no. 1, p. 2124, 2019.
[7] E. Kazemivalipour et al., "Reconfigurable MRI technology for low-SAR imaging of deep brain stimulation at 3T: Application in bilateral leads, fully-implanted systems, and surgically modified lead trajectories," Neuroimage, vol. 199, pp. 18-22, 2019.
[8] L. Golestanirad et al., "Reconfigurable MRI coil technology can substantially reduce RF heating of deep brain stimulation implants: First in-vitro study of RF heating reduction in bilateral DBS leads at 1.5 T," PLoS One, vol. In Press, 2019.
[9] P.-S. Wei, B. Yang, C. McElcheran, L. Golestanirad, and S. J. Graham, "Reducing Radiofrequency-induced Heating in Realistic Deep Brain Stimulation Lead Trajectories using Parallel Transmission," Proc. Int. Soc. Magn. Reson. Med., vol. 26, 2018.
[10] C. E. McElcheran, B. Yang, K. J. Anderson, L. Golestanirad, and S. J. Graham, "Parallel radiofrequency transmission at 3 tesla to improve safety in bilateral implanted wires in a heterogeneous model," Magnetic resonance in medicine, vol. 78, no. 6, pp. 2406-2415, 2017.
[11] C. McElcheran et al., "Low Heating B1 Mapping in Parallel Transmit for Deep Brain Stimulators," Proc. Intl. Soc. Mag. Reson. Med., vol. 25, 2017.
[12] C. McElcheran et al., "Parallel Transmission for Heating Reduction in Realistic Deep Brain Stimulation Lead Trajectories," Proc. Intl. Soc. Mag. Reson. Med. , vol. 25, 2017.
[13] L. Golestanirad, B. Keil, L. M. Angelone, G. Bonmassar, A. Mareyam, and L. L. Wald, "Feasibility of using linearly polarized rotating birdcage transmitters and close‐fitting receive arrays in MRI to reduce SAR in the vicinity of deep brain simulation implants," Magnetic resonance in medicine, vol. 77, no. 4, pp. 1701-1712, 2017.
[14] L. Golestanirad et al., "Construction and modeling of a reconfigurable MRI coil for lowering SAR in patients with deep brain stimulation implants," Neuroimage, vol. 147, pp. 577-588, 2017.
[15] C. McElcheran, L. Golestanirad, and S. Graham, "Heating Reduction in Unilateral And Bilateral Implanted Leads At 3T Using Parallel Radiofrequency Transmission in a Heterogeneous Head Model," Proc. Intl. Soc. Mag. Reson. Med., vol. 24, 2016.
[16] L. Golestanirad et al., "A Patient-adjustable MRI coil for implant-friendly imaging of deep brain stimulation: Design, construction, and patient-specific numerical simulations," Proc. Intl. Soc. Mag. Reson. Med. 24, 2016.
[17] Clare McElcheran, L. Golestanirad, and S. Graham, "Reduced Heating of Implanted Electrical Conductors Using Parallel Radiofrequency Transmission," Proc. Intl. Soc. Mag. Reson. Med. 22 2014.
[18] L. Golestanirad et al., "Changes in the specific absorption rate (SAR) of radiofrequency energy in patients with retained cardiac leads during MRI at 1.5 T and 3T," Magnetic resonance in medicine, vol. 81, no. 1, pp. 653-669, 2019.
[19] L. Golestanirad et al., "RF heating of deep brain stimulation implants in open-bore vertical MRI systems " Magnetic resonance imaging, vol. InPress, 2019.
[20] L. Golestanirad et al., "Reducing RF-Induced Heating Near Implanted Leads Through High-Dielectric Capacitive Bleeding of Current (CBLOC)," IEEE Transactions on Microwave Theory and Techniques, vol. 67, no. 3, pp. 1265-1273, 2019.
[21] L. Golestanirad, L. L. Wald, B. Keil, and G. Bonmassar, "Reducing heating of implanted leads through High-Dielectric Capacitive Bleeding of Current (HD-CBLOC): concepts, simulations and experimental results," Proc. Intl. Soc. Mag. Reson. Med., vol. 26, 2018.
[22] C. Bulkes and S. Denker, "Mri compatible implanted electronic medical device and lead US Patent US8255054B2," 2007.
[23] E. Atalar, J. Allen, P. Bottomley, W. Eldelstein, and P. V. Karmarkar, "MRI-safe high impedance lead systems US Patent US8688226B2," 2006.
Figures