4175
Simulation strategies to assess RF heating of implants: Do we need a paradigm shift?1Department of Radiology, Northwestern University, Chicago, IL, United States, 2Department of Neurosurgery, Albany Medical Center, Albany, NY, United States
Synopsis
Numerical computation of radio-frequency (RF) fields in human body with implants is currently the only practical approach to obtain reliable estimation of the specific absorption rate (SAR) for safety assessment. Here we examine how detailed the model of a human body with a deep brain stimulation (DBS) implant needs to be to provide predictions of worst-case SAR scenarios for safety assessments.
Introduction
Predicting radiofrequency (RF) heating of medical implants in MRI environment is notoriously complex [1], yet has become increasingly in demand [2]. Currently, numerical simulation of RF fields in human body is the only practical approach to obtain a reliable estimation of local SAR and by proxy, RF heating in the tissue around a medical implant [3]. Nevertheless, performing simulations with the dynamic spatial resolution that spans details of implant geometry (~500µm), multi-compartment body models (~1mm), and MRI equipment (~1m) is still impractical even with the latest advancement in computing technology. Using body models with large number of tissue classes has been increasingly adopted at the expense of dramatically simplifying implant’s geometry. Here we show, for the first time, that this approach gives a false sense of accuracy, as the error introduced by simplifying implant’s geometry goes well beyond the benefit of using a realistic body model. We systematically studied the impacts of different parameters / assumptions (i.e., dielectric properties of tissue, complexity of body model, truncation of body model, and internal geometry of the implant) on prediction of SAR values in a patient with a deep brain stimulation implant undergoing MRI at 1.5 T and 3 T. The major goal was to find a compromise allowing simplifications of the model while maintaining confidence in prediction of worst-case SAR scenarios and therefore to provide an initial reliable platform for MRI safety assessment in this specific patient population.Methods
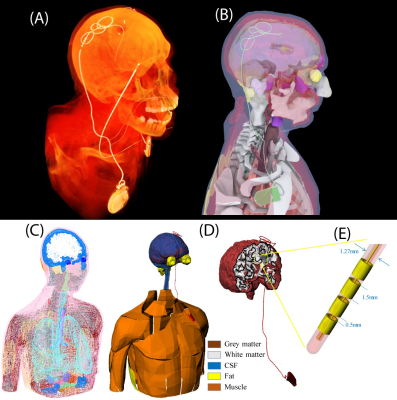
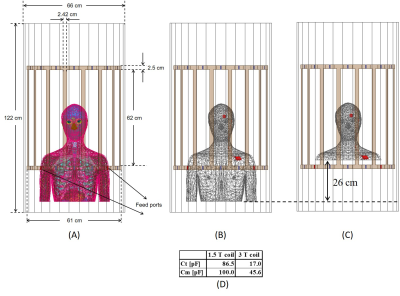
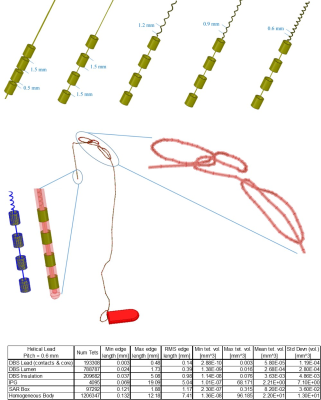
A multi-compartment body model with a fully implanted DBS device was constructed based on realistic device configuration extracted from CT images of a patient (Figure 1). We assessed the sensitivity of SAR around the tip of DBS lead to variation in conductivities of body tissues during MRI at 1.5 T and 3T by systematically changing the conductivity of five major tissues surrounding the device, namely grey matter (0.3 S/m→0.7 S/m), white matter (0.18 S/m→0.42 S/m), CSF (1.2 S/m→2.8 S/m), fat (0.018 S/m→0.042 S/m) and muscle (0.4 S/m→1 S/m) while keeping the conductivity of all other tissues constant (Figure 1). In the next step we assessed whether a homogenous body model could predict the worst-case SAR scenario as predicted by the heterogenous body model. To do this, we assigned the averages permittivity of the whole tissue set to a homogeneous body model while varying its conductivity from 0.01 S/m→1 S/m covering a range of low to high tissue conductivities. We also investigated the effect of truncating the body model right below the implanted pulse generator (IPG) (Figure 2). Finally, we evaluated the effect of incorporating realistic geometric features of the implant such as tightly-coiled helical wires connecting electrode contacts on predicted SAR values (Figure 3). All simulations were implemented in ANSYS Electronics Desktop (ver. 2019.1.0) on a server with 1.5TB Memory and 2×Xenon(R) Gold 6140 CPUs each having 32 processing cores and maximum of 1g-averaged SAR (MaxSAR1g) in a 2cm×2cm×2cm cubic area around the tip was calculated.Results
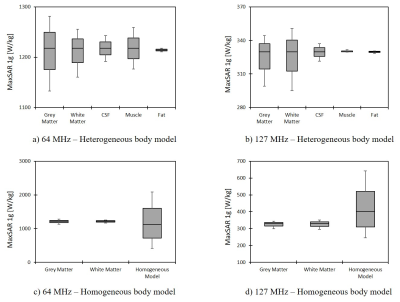
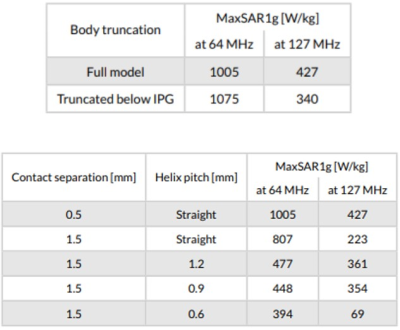
Simulation times and memory requirement for models with heterogeneous and homogeneous bodies were 10 hours 35 mins and 438 GB, and 5 hours 50 mins and 367 GB, respectively. In models with heterogeneous body, the variation in conductivity of the gray matter and white matter generated the largest variation in SAR amounting to 13% in 1.5T and 19% in 3T (Figure 4). The homogeneous body model with conductivities varied in a range of low to high values for tissue (0.01 S/m→1 S/m) covered the full range of SAR values predicted by the heterogeneous body model (Figure 4). Truncating the homogeneous model below the IPG changed the SAR by 7% at 64 MHz, and by 20% at 127 MHz (Figure 5), while saving 250% in simulation time (1 hour 40 mins) and 148% in memory allocation (148 GB). Despite allocating high computational resources to simulations (1.5 TB RAM, 64 processors), realistic features of implant geometry such as coiled interconnections could not be incorporated in simulations with heterogeneous body model. Incorporating these features in the homogeneous model by replacing the straight core wire with helical interconnecting wires of different pitch (1.2 mm, 0.9 mm and 0.6 mm) led to a significant change in SAR, amounting to 50% at 64 MHz and 70% at 127 MHz (Figure 5). Varying the spacing between electrode contacts changed the SAR by 20% at 64 MHz and by 48% at 127 MHz.Conclusion
Increasing number of applications rely on prediction of RF heating of implants including MRI hardware developments [4-13,17,18], protocol optimization [14], and implant design [15,16]. High resolution body models originally developed to evaluate MRI fields in human body at ultra-high fields are suggested to assess RF heating of implants, but this has been only possible with the tradeoff of simplifying implant geometry. Our results show that such trade-offs can introduce errors that negate the original premise of using realistic body models. As long as local SAR amplification around the implant is of interest, using simplified body models with a range of typical tissue properties and a detailed realistic implant geometry provides a more reliable estimation of worst-case SAR values than a heterogeneous body model in conjunction with a simplified implant model.Acknowledgements
This work has been supported by NIH grants R00EB021320, R03EB025344, and R03EB024705.References
[1] Mattei, E., Triventi, M., Calcagnini, G., Censi, F., Kainz, W., Mendoza, G., . . . Bartolini, P. Complexity of MRI induced heating on metallic leads: experimental measurements of 374 configurations. Biomedical engineering online 7, 11 (2008).
[2] Scanning patients with MR-conditional implants. https://www.usa.philips.com/healthcare/education-resources/publications/fieldstrength/mri-and-mr-conditional-implants.
[3] U.S. Food and Drug Administration “Reporting of Computational Modeling Studies in Medical Device Submissions - Draft Guidance for Industry and Food and Drug Administration Staff” http://www.fda.gov/RegulatoryInformation/Guidances/ucm371016.htm Date of Access 12/15/2015
[4] McElcheran, C., Golestanirad, L., Iacono, M., Wei, P.-S., Yang, B., Anderson, K., . . . Graham, S. Numerical Simulations of Realistic Lead Trajectories and an Experimental Verification Support the Efficacy of Parallel Radiofrequency Transmission to Reduce Heating of Deep Brain Stimulation Implants during MRI. Nature Scientific Reports 9, 2124 (2019).
[5] Kazemivalipour, E., Keil, B., Vali, A., Rajan, S., Elahi, B., Atalar, E., . . . Golestanirad, L. Reconfigurable MRI technology for low-SAR imaging of deep brain stimulation at 3T: Application in bilateral leads, fully-implanted systems, and surgically modified lead trajectories. Neuroimage 199, 18-22 (2019).
[6] Golestanirad, L., Lampman, D., Kazemivalipour, E., Habara, H., Atalar, E., Rosenow, J., . . . Kirsch, J. RF heating of deep brain stimulation implants in open-bore vertical MRI systems Magnetic resonance imaging InPress (2019).
[7] Golestanirad, L., Kirsch, J., Bonmassar, G., Downs, S., Elahi, B., Martin, A., . . . Wald, L. L. RF-induced heating in tissue near bilateral DBS implants during MRI at 1.5 T and 3T: The role of surgical lead management. NeuroImage 184, 566-576 (2019).
[8] Golestanirad, L., Kazemivalipour, E., Keil, B., Downs, S., Kirsch, J., Elahi, B., . . . Wald, L. L. Reconfigurable MRI coil technology can substantially reduce RF heating of deep brain stimulation implants: First in-vitro study of RF heating reduction in bilateral DBS leads at 1.5 T. PLoS One 14 (2019). [9] McElcheran, C. E., Yang, B., Anderson, K. J., Golestanirad, L. & Graham, S. J. Parallel radiofrequency transmission at 3 tesla to improve safety in bilateral implanted wires in a heterogeneous model. Magnetic resonance in medicine 78, 2406-2415 (2017).
[10] McElcheran, C., Golestanirad, L., Iacono, M., Yang, B., Anderson, K., Bonmassar, G. & Graham, S. J. Low Heating B1 Mapping in Parallel Transmit for Deep Brain Stimulators. Proc. Intl. Soc. Mag. Reson. Med. 25 (2017).
[11] McElcheran, C., Golestanirad, L., Iacono, M., Yang, B., Anderson, K., Bonmassar, G. & Graham, S. Parallel Transmission for Heating Reduction in Realistic Deep Brain Stimulation Lead Trajectories. Proc. Intl. Soc. Mag. Reson. Med. 25 (2017).
[12] Golestanirad, L., Keil, B., Angelone, L. M., Bonmassar, G., Mareyam, A. & Wald, L. L. Feasibility of using linearly polarized rotating birdcage transmitters and close‐fitting receive arrays in MRI to reduce SAR in the vicinity of deep brain simulation implants. Magnetic resonance in medicine 77, 1701-1712 (2017).
[13] Golestanirad, L., Iacono, M. I., Keil, B., Angelone, L. M., Bonmassar, G., Fox, M. D., . . . Mareyam, A. Construction and modeling of a reconfigurable MRI coil for lowering SAR in patients with deep brain stimulation implants. Neuroimage 147, 577-588 (2017).
[14] Golestanirad, L., Rahsepar, A. A., Kirsch, J. E., Suwa, K., Collins, J. C., Angelone, L. M., . . . Wald, L. L. Changes in the specific absorption rate (SAR) of radiofrequency energy in patients with retained cardiac leads during MRI at 1.5 T and 3T. Magnetic resonance in medicine 81, 653-669 (2019).
[15] Golestanirad, L., Angelone, L. M., Kirsch, J., Downs, S., Keil, B., Bonmassar, G. & Wald, L. L. Reducing RF-Induced Heating Near Implanted Leads Through High-Dielectric Capacitive Bleeding of Current (CBLOC). IEEE Transactions on Microwave Theory and Techniques 67, 1265-1273 (2019).
[16] Golestanirad, L., Angelone, L. M., Iacono, M. I., Katnani, H., Wald, L. L. & Bonmassar, G. Local SAR near deep brain stimulation (DBS) electrodes at 64 MHz and 127 MHz: A simulation study of the effect of extracranial loops Magnetic Resonance in Medicine 88, 1558-1565 (2016).
[17] McElcheran, C., Golestanirad, L. & Graham, S. Heating Reduction in Unilateral And Bilateral Implanted Leads At 3T Using Parallel Radiofrequency Transmission in a Heterogeneous Head Model. Proc. Intl. Soc. Mag. Reson. Med. 24 (2016).
[18] Golestanirad, L., Keil, B., Ida-Iacono, M., Bonmassar, G., Angelone, L. M., LaPierre, C. & Wald, L. L. A Patient-adjustable MRI coil for implant-friendly imaging of deep brain stimulation: Design, construction, and patient-specific numerical simulations. Proc. Intl. Soc. Mag. Reson. Med. 24 (2016).
Figures