4174
Analysis of changes in magnetic susceptibility artifacts due to static magnetic field strength, imaging sequence and chemical composition
Ryusuke Nakai1, Seiji Yamaguchi2, Mitsuaki Toda3, Takashi Azuma4, and Hiroo Iwata4
1Kokoro Research Center, Kyoto University, Kyoto, Japan, 2College of Life and Health Sciences, Chubu University, Aichi, Japan, 3Graduate School of Health Care Sciences, Jikei Institute, Osaka, Japan, 4Graduate School of Medicine, Kyoto University, Kyoto, Japan
1Kokoro Research Center, Kyoto University, Kyoto, Japan, 2College of Life and Health Sciences, Chubu University, Aichi, Japan, 3Graduate School of Health Care Sciences, Jikei Institute, Osaka, Japan, 4Graduate School of Medicine, Kyoto University, Kyoto, Japan
Synopsis
Reducing or resolving susceptibility artifacts is important for development of materials for medical implants. In this study, we measured and evaluated magnetic susceptibility artifacts for various metals and ceramics using two MRI systems with different static magnetic field strengths and multiple imaging sequences. MR images were acquired following the criteria of the spin echo sequence and gradient echo sequence required in the US FDA standard artifact evaluation test. The results were used to clarify the relationships among artifact size in MR images, volume magnetic susceptibility of samples, and static magnetic field strength of the MRI system.
Purpose
Magnetic resonance imaging (MRI) permits noninvasive diagnosis of human disease. Adjustment of the sequence and parameters of MRI can yield morphological and functional images of varying contrast. The importance of MRI in diagnostic imaging has increased yearly. However, in patients with medical devices made of metal or including metallic parts, such as stents, radiation markers and emboli coils, artifacts associated with magnetic susceptibility may occur on MR images. This often makes it difficult to evaluate tissues around such medical devices and to use MRI for follow-up of patients after device implantation. Therefore, reducing or resolving susceptibility artifacts is important in development of materials for medical implants. In this study, we measured and evaluated magnetic susceptibility artifacts for various metals and ceramics using two MRI systems with different static magnetic field strengths and multiple imaging sequences. This study provides useful information for development of artifact-reducing materials for medical implants.Materials and Methods
Metals (Ag, Al, Cu, Mg, Nb, Sn, Ta, Ti, Zr) and ceramics (Al2O3, CuO, Nb2O5, SiO2, Ta2O5, TiO2, ZrO2) were prepared as materials for MRI tests. The size of all samples to be imaged was a φ10.0 mm × 0.5 mm cylinder (disk shape). Metal samples were cut from a metal rod (φ10.0 mm) using a precise cutting machine with a diamond blade. For the ceramic samples, ceramic pellets were molded by a mold press machine and pressed at 392 MPa using a CIP (Cold Isostatic Pressing) machine. The samples were polished with a diamond file to adjust the size, after they were sintered in an electric furnace at 1450°C for 4 hours. In artifact evaluation experiments, disk-shaped samples were fixed with agarose gel in a test tube and imaged (Fig. 1). MRI was performed on a 1.5 T whole body scanner (Magnetom Sonata, Siemens AG, Erlangen, Germany) and a 3.0 T whole body scanner (Magnetom Verio, Siemens AG) using dedicated small-bore phased array receiver coils (Takashima Seisakusho Co., Ltd., Tokyo, Japan) (Fig. 2) for high resolution MRI. MR images were acquired using a spin echo sequence (TR, 500 ms; TE, 20 ms; pixel size, 0.5 x 0.5 mm; thickness, 2.0 mm; matrix size, 256 x 256; NEX, 2) and a gradient echo sequence (TR, 250 ms; TE, 15 ms; pixel size, 0.4 x 0.4 mm; thickness, 2.0 mm; flip angle, 30 deg.; matrix size, 320 x 320; NEX, 2), using the parameters required in the US FDA standard artifact evaluation test [1]. The artifact length from the sample margin was measured in the acquired images using in-house software for artifact evaluation. The sizes of artifacts in the images were evaluated to determine the effects of static magnetic field strength, imaging sequence, and magnetic susceptibility.Results and Discussion
Examples of MR images taken with spin echo and gradient echo sequences using a 1.5 T MRI system and a dedicated small-bore phased array receiver coil are shown in Fig. 3. For pure copper, which has a susceptibility approximately equal to that of water and human tissue (≈ -9 ppm), the form depicted was approximately a slender rectangle with both sequences. For pure Ti, which has a different susceptibility to that of water, smile-type artifacts were noted with the spin echo sequence and large butterfly-type artifacts were seen with the gradient echo sequence. The relationship between artifact size and volume magnetic susceptibility of metal samples when imaged with a 1.5 T MRI system and a dedicated small-bore phased array receiver coil is shown in Fig. 4. The artifact length was correlated with the measured magnetic susceptibility of the metal samples. The artifact size decreased as the volume magnetic susceptibility became close to that of the surrounding material (agarose gel, water ≈ -9 ppm). The artifact length increased in the following order: spin echo (1.5 T) < spin echo (3.0 T) < gradient echo (1.5 T) < gradient echo (3.0 T). The artifact on the 3.0 T MRI system was 2.3 times larger than that on the 1.5 T MRI system, and the artifact with the gradient echo was 3.3 times larger than that with the spin echo. In ceramic samples, ZrO2 had very small artifacts with both sequences, but TiO2 had moderate artifacts. These results show that even similar ceramics can have different artifact sizes, which indicates the importance of measuring the magnetic susceptibility of ceramics.Conclusion
The relationship between material composition and magnetic susceptibility artifacts using various metal and ceramic samples was investigated in this study. In metal samples, the artifact size decreased as the volume magnetic susceptibility became close to that of the surrounding material. The artifact size was larger on 3.0 T MRI than on 1.5 T MRI and larger with a gradient echo sequence than with a spin echo sequence. In ceramic samples, similar ceramics had different artifact sizes. The results of this study provide important information for development of materials for use in implantable medical devices.Acknowledgements
This study was conducted using the MRI scanner and related facilities at the Institute for Frontier Life and Medical Sciences and Kokoro Research Center (Kyoto University, Kyoto, Japan). This work was supported by the Japan Society for the Promotion of Science (JSPS; KAKENHI grant numbers JP19H05366 & JP19K22963).References
[1] ASTM F2119, Annual Book of ASTM Standards, Vol. 13.01.Figures

Fig. 1. The
disk-shaped sample was fixed with agarose gel in a test tube.

Fig. 2. The
dedicated small-bore phased array receiver coil.
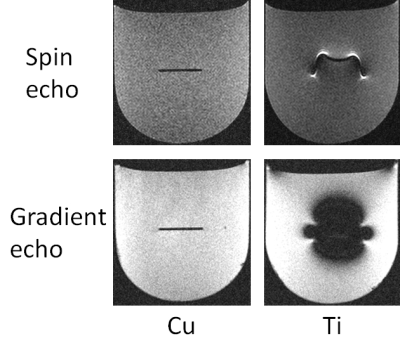
Fig. 3. Examples of MR images taken with
spin echo and gradient echo sequences using a 1.5 T MRI system and a dedicated
small-bore phased array receiver coil.
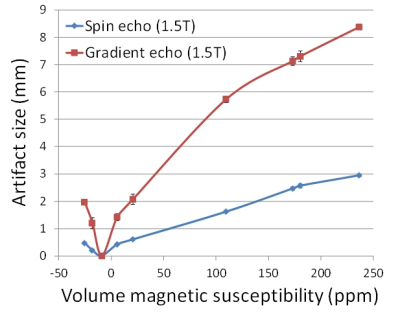
Fig. 4. Relationship between artifact size
and volume magnetic susceptibility of metal samples when imaged with a 1.5 T MRI
system and a dedicated small-bore phased array receiver coil.