4172
A Framework for Worst-Case SAR Reduction when Controlling Induced Currents on Elongated Conductors using Parallel Transmit1Biomedical Engineering Department, School of Biomedical Engineering & Imaging Sciences, King's College London, London, United Kingdom, 2Centre for the Developing Brain, School of Biomedical Engineering & Imaging Sciences, King's College London, London, United Kingdom
Synopsis
MRI with elongated conductors (“wires”), risks tissue heating and reduced image quality. Parallel Transmit (pTx) systems can be configured to generate useful magnetic fields while reducing currents on wires, provided suitable current sensing is achieved. We propose a framework based on a current mode analysis for determining worst case SAR (wcSAR) when driving a pTx array so as to remove high current modes. This approach is demonstrated using FDTD simulations of two 8 channel pTx coils allowing comparison of full knowledge of currents with restricted point measurement, systematically determining the impact of current sensor number and location on wcSAR control.
Introduction
MRI with elongated conductors (‘wires’) risks intense tissue heating due to induced currents produced by coupling with the radiofrequency (RF) system. Parallel Transmit (pTx) systems can allow induced currents to be controlled if the coupling between individual channels and the wire can be determined. In situ current measurement can be used1-3, but only data at sensor locations is then available, providing incomplete information. For an n channel pTx system and m sensors it is always possible to identify n-m orthogonal drive configurations that cause the current at all the sensor locations to be zero, which could result in reduced risk of heating. In this study we developed and used a worst-case estimation framework combined to explore the safety implications of deploying different numbers of sensors and the impact of their placement along the wire.Methods
FDTD simulation (Sim4Life, ZMT, Zurich) provides a matrix of current distributions (J) and fields resulting from the interaction between a wire and a pTx RF array coil. Singular value decomposition (SVD) of J yields singular vectors that induce both high currents (coupling modes - high singular values) and low currents (‘null’ modes - low to zero singular values). SAR is quantified using Q-matrices4 compressed into virtual observation points (VOP)5. Worst-case SAR (wcSAR) can be obtained from the maximum eigenvalue of the resulting VOPs.Reduction of currents is achieved by removing the coupling modes from the system. This mode removal can be understood by projecting the VOP matrices ($$$\mathbf{A}$$$) into the null-space obtained from the SVD ($$$\mathbf{V}^{0}$$$);
$$\mathbf{A}^{0}\,=\,\mathbf{V}^{0}\,\mathbf{A}\,\mathbf{V}^{0*}$$
and the maximum eigenvalues of these matrices ($$$\mathbf{A}^{0}$$$) after projection then specifies the new wcSAR.
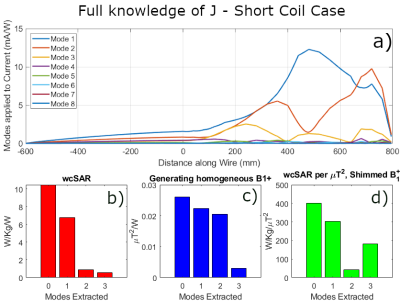
Removal of degrees of freedom also limits the ability of the coil to make RF magnetic fields (B1+). To account for this, RF shimming is performed after modes are removed6 and the wcSAR values are normalised to the square of the average B1+ in a chosen imaging region and scaled by the power needed to achieve it.
Two 8-channel Tx/Rx cardiac RF coils (one short, one long) were simulated on the Duke model (IT'IS ViP3.1) at 64MHz, with a realistic cardiac guidewire wire (Fig.1). The wire (core:∅ = 1mm; insulating layer: 0.75mm thickness) had its distal 5mm stripped of insulation. To reduce computational time, Sim4Life’s Huygens Box method was applied. MATLAB was used for all following analysis.
J was sampled along the wire to simulate individual current sensing positions. The above analysis was performed using either the full current distribution or using one, two and three sensing positions, exploring all combinations ranging from 100mm outside the body to the wire tip.
Results & Discussion
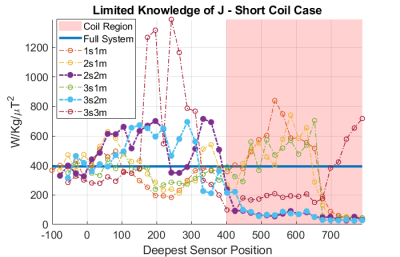
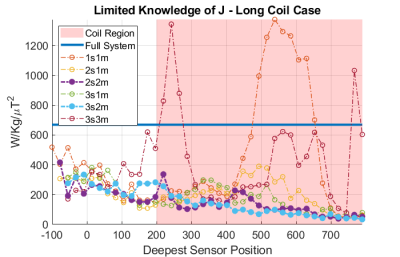
Fig.2 shows the framework assuming full knowledge of J. Fig.2a) indicates that there are three dominant coupling modes. Removing the first two of these does successfully reduce wcSAR. However, when working a constant B1+, removing the third coupling mode from the system actually increases wcSAR because the reduction in current generation/unit input power is more than offset by the need to increase the drive power to compensate for the loss of efficiency for B1+ generation(Fig.2d).If only spot current measurements are available, then the modes that can actually be inferred from the available data may become quite different from the true modes, leading to different outcomes. Fig.3 shows the normalised wcSAR reduction as a function of modes removed from the system and sensing positions (number and location) for the short coil. With limited knowledge of J, removal of one coupling mode consistently reduces wcSAR below 50% of its full system value when the current sensing position is near the wire tip. Removing two modes provides consistent reduction provided the deepest sensor is within the geometrical volume enclosed by the RF coil, where the RF electric fields are the strongest. Removing a third coupling mode is not beneficial, as was the case with full knowledge of J. In Fig.4 we can see similar behaviour for the long coil.
Conclusion
Previous work has shown1 that for a complex wire geometry, at least two sensing positions were needed to successfully reduce heating through induced currents. Here, a framework built to assess the impact on worst-case SAR of seeking to control the current with either full or only limited knowledge of current distribution was introduced and tested on two simulated scenarios. For both 8 channel cases, regardless of having full or limited knowledge of J, no more than two dominant coupling modes should be removed from the system, otherwise the remaining degrees of freedom are insufficient to operate as efficient B1+ generators. This requires at least two current sensing positions to be used and this cannot reliably be achieved if these are located remotely from the coil elements.The proposed framework can be applied to a wide class of problems in which pTx is to be deployed to perform MRI while seeking to control currents on conducting wires. It allows for quick comparisons between scenarios and for identifying behavioural patterns of scenarios that would otherwise remain hidden if only arbitrary sensing positions were chosen.
Acknowledgements
JNT is funded by MRC CASE studentship [MR/P01674X/1]. This work was supported by the Wellcome EPSRC Centre for Medical Engineering at Kings College London [WT 203148/Z/16/Z], MRC strategic grant [MR/K006355/1], MRC development pathways funding scheme [MR/N027949/1], and by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.References
1. Etezadi-Amoli M, Stang P, Kerr A, Pauly J, Scott G. Controlling radiofrequency-induced currents in guidewires using parallel transmit. Magn Reson Med. 2015;74(6):1790-1802. doi:10.1002/mrm.25543.
2. Teixeira J. N., et al. Exploring the impact of nulling currents on a cardiac guidewire in reducing worst case SAR at 1.5T. In Proceedings of the 26th Annual Meeting of ISMRM, Paris,France, 2018. Abstract 0299
3. Teixeira J. N., et al. Planning Current Nulling for Worst Case SAR reduction in a Cardiac Guidewire Scenario at 1.5T. In Proceedings of the 27th Annual Meeting of ISMRM, Montreal, Canada, 2019. Abstract 4179
4. Graesslin, I., Homann, H., Biederer, S., Börnert, P., Nehrke, K., Vernickel, P., Mens, G., Harvey, P. and Katscher, U. A specific absorption rate prediction concept for parallel transmission MR. Magn Reson in Med. 2012; 68(5):1664-1674. doi: 10.1002/mrm.24138
5. Eichfelder, G. and Gebhardt, M. Local specific absorption rate control for parallel transmission by virtual observation points. Magn Reson in Med. 2011; 66(5): 1468-1476. doi: 10.1002/mrm.22927
6. Setsompop K, Wald LL, Alagappan V, Gagoski BA, Adalsteinsson E. Magnitude least squares optimization for parallel radio frequency excitation design demonstrated at 7 Tesla with eight channels. Magn. Reson. Med. 2008; 59: 908–915.
Figures
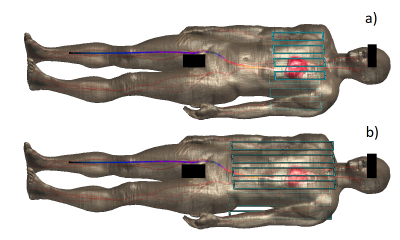
Figure 1 - Visual representation of the two scenarios simulated with Sim4Life. Region used for the shimming algorithm (heart) in red. Wires show current distributions induced by one element of the coil. a) short coil - width: 275mm, length: 250mm, 8 elements, 3 capacitors per element; b) long coil - width: 275mm, length: 500mm, 8 elements, 3 capacitors per element.