4163
Magnetic field stimuli improve hepatic delivery of oxaliplatin to colorectal liver metastatic tumor bearing rats
Venkateswara Gogineni1, Dilip Maddirela1, Woo Ram Park2, Jaidip Jagtap1, Abdul Parchur1, Gayatri Sharma1, El-Sayed H Ibrahim1, Amit Joshi1, Andrew Larson2, Dong-Hyun Kim2, and Sarah B White1
1Medical College of Wisconsin, Milwaukee, WI, United States, 2Northwestern University, Chicago, IL, United States
1Medical College of Wisconsin, Milwaukee, WI, United States, 2Northwestern University, Chicago, IL, United States
Synopsis
Systemic chemotherapy is the standard of care for the treatment of unresectable metastatic colorectal cancer. However, new drug delivery platforms are needed to mitigate its associated toxicities. In this study, we develop and evaluate a liposome formulation that deliver oxaliplatin under magnetic field stimulus site selectively release in high concentration to alleviate the off-target effects in a rat model of colorectal liver metastases (CRLM). Quantitative MRI enabled assessment of changes in tumor characteristics over time for confirmation of procedural success and proper catheter-selective tumor targeting, which allows for high concentrations of oxaliplatin and improves survival outcomes in CRLM tumor-bearing rats.
Introduction
The American cancer society estimates that in the United States, colorectal cancer is the third leading cause of cancer-related deaths in men and in women, and the second most common cause of cancer deaths when men and women are combined. Systemic chemotherapy is the standard of care for the treatment of unresectable metastatic colorectal cancer. However, new drug delivery platforms are needed to mitigate the associated severe systemic toxicities. The aim of this study was to develop and evaluate a liposome formulation that deliver oxaliplatin under magnetic field stimulus site selectively release in high concentration to alleviate the off-target effects in a rat model of colorectal liver metastases (CRLM). Our approach integrated nanomedicine, molecular MRI, optical imaging, site selective delivery and controlled release to optimize treatment efficacy.Methods
Hybrid liposome-magnetic nanoparticles loaded with Cy5.5 dye and oxaliplatin (L-NIR- Fe3O4/OX) were fabricated by using the thermal decomposition method. CRLM (CC-531) cell viability was assessed and rats orthotopically implanted with CC-531 cells were treated with L-NIR- Fe3O4/OX or by drug alone via different routes of administration, up to 3 cycles of alternating magnetic field (AMF). Wistar rats (n=4), weighing 300-350g, were used for in-vivo scans. For the inoculation procedure, anesthesia was obtained using 2-3% isoflurane. A 3-cm midline incision was made and both the left and right hepatic lobes were mobilized to implant sub-capsular tumors. Approximately two weeks post-implantation, X-ray digital subtraction angiography was performed to selectively place a catheter into tumor feeding branches of the hepatic artery. After catheter placement, 500μL of liposomes were infused, after which the catheter was withdrawn. Optical and MR imaging was performed to assess the targeted delivery. Biodistribution and histology was performed to determine the distribution of oxaliplatin. MRI was performed before and immediately after liposomes infusion, as well as after AMF triggering using T2* imaging sequence using the following imaging parameters: repetition time (TR) = 800 ms, 9 echo times (TE) ranging from 4 ms to 48 ms in 5.5 ms increments, flip angle = 50⁰, matrix = 128x128, field of view (FOV) = 45x45 mm2, slice thickness =1 mm, acquisition bandwidth = 586 Hz/pixel, # averages = 2, and scan time ~1 minute/slice. Stacks of parallel axial and coronal slices covering the liver were acquired using respiratory gated acquisition to avoid breathing artifacts. T2* maps were created for all acquired slices by fitting signal intensities from the nine echoes to mono-exponential decaying curves on the pixel level. T2* values were measured in the tumors and normal tissues in all slices at three timepoints: before contrast infusion, after infusion, and after AMF triggering.Results
L-NIR- Fe3O4/OX presented an increase of oxaliplatin release (~18%) after the samples were exposed to the AMF. Cell viability after L-NIR- Fe3O4/OX treatment and exposure to AMF were significantly lower than those not exposed to AMF (P < .001). Optical imaging showed a significant release of oxaliplatin in vitro and in vivo among mesenteric vein (MV) injected group of animals. MRI showed that T2* values in the tumors (13.8±0.8 ms) were significantly (P = .0004) different from those in normal liver tissues (4.7±0.7 ms). The tumors’ T2* values significantly (P = .003) decreased post contrast infusion to 12±0.6 ms. T2* further decreased after AMF triggering to 11.3±0.8 ms, which was not significantly different from the values before AMF triggering (P = .2). Biodistribution analysis showed significantly higher levels of oxaliplatin in liver tissues compared to lungs (P < .001) and intestines (P < .001) in the MV animals that received AMF after L-NIR- Fe3O4/OX administration. Large tumor necrotic zones and significant improvement in the survival rates were noted in the MV animals treated with AMF.Discussion
This study showed the capability of noninvasive assessment of liposome nanoparticles distribution to liver tumors using MRI imaging. Quantitative MRI enabled assessment of changes in tumor T2* values over time for confirmation of procedural success and proper catheter-selective tumor targeting. The presence of iron oxide in the liposomes allows for triggered release of therapeutic agents by external triggers like alternating magnetic field or heat. Stimulated drug release reduces off-target effects and improves the efficiency of local high dose release. Further, the amount of therapeutic agent delivered to the targeted region can be quantitated and the outcome of the procedure can be assessed.Conclusion
In conclusion, site selective delivery allows for high concentrations of oxaliplatin and improves survival outcomes in colorectal liver metastasis tumor bearing rats.Acknowledgements
No acknowledgement found.References
- American Cancer Society. Colorectal Cancer Facts & Figures 2017-2019.
- Guardia P et al. ACS nano, 2012. 6(4): p. 3080-3091.
- Bae KH et al. ACS nano, 2012. 6(6): p. 5266-5273.
- Mikhaylov G et al. Nature nanotechnology, 2011. 6(9): p. 594-602.
- Tansi, FL et al. Small, 2013. 9(21): p. 3659-3669.
Figures
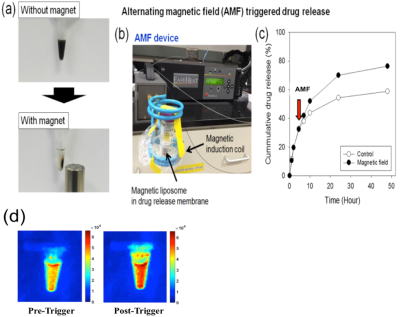
Figure 1.
(a) Photographs of the magnetic separation of magnetic liposome,
(b) Photograph of AMF induction device, (c) Cumulative oxaliplatin release when
exposed (or not) to a high frequency AMF. (d) NIR fluorescence imaging was performed twice, before and after triggering of
external magnetic field, on magnetic nanoparticle embedded Cy5.5 (NP-Cy5.5) complex system (λex/λem=663nm/longpass
700nm) at 100 ms exposure time.
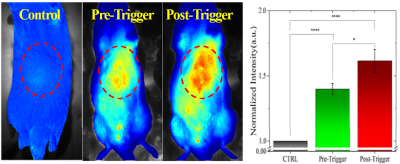
Figure 2. Cy5.5 imaging in vivo Average intensity from ROI (red
dotted circles in plot exhibits significant enhancement in fluorescence signal
due to release in Cy5.5 post-triggering. (n>5; each group).
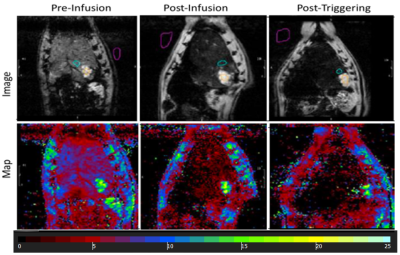
Figure 3. T2* maps drawn on MR image showed signal loss with liposome
delivery (Fe) deposition in tumor region (colored green) and a further loss in
signal was observed with AMF triggering (T2* values measured in ms).