4162
In vivo MRI measurement of microstructural constraints for direct drug delivery within the brain1Neuroradiology Unit and CERMAC, Vita-Salute San Raffaele University, IRCCS San Raffaele Scientific Institute, Milan, Italy, 2Department of Electronics, Information and Bioengineering, Politecnico di Milano, Milan, Italy, 3Department of Medical Biotechnology and Translational Medicine, Università degli Studi di Milano, Milan, Italy, 4Neurosurgical Oncology Unit, IRCCS Humanitas Clinical and Research Center, Rozzano (MI), Italy, 5Department of Mechanical Engineering, Imperial College London, London, United Kingdom, 6Department of Veterinary Medicine, Università degli Studi di Milano, Milan, Italy, 7Department of Health, Animal Science and Food Safety, Faculty of Veterinary Medicine, Università degli Studi di Milano, Milan, Italy, 8Philips Healthcare, Milan, Italy, 9Department of Oncology and Hemato-Oncology, Università degli Studi di Milano, Milan, Italy
Synopsis
Brain tissue microstructure may influence the efficient delivery of therapeutics within the brain. Diffusion Tensor Imaging (DTI) enables the depiction of tissue properties in vivo, and thus is potentially relevant for planning convection-enhanced delivery (CED) within the brain. We report on the quantitative assessment of the distribution of a Gadolinium solution infused by CED within the brain of a live ovine model. Infusate distributions were measured at multiple timepoints and compared to microstructural properties as depicted by DTI, thus demonstrating the impact of tissue features and catheter positioning on drug distribution in vivo.
INTRODUCTION
Targeted delivery of therapeutics within the brain represents an innovative approach for personalized treatments. CED is effective in bypassing the blood-brain barrier and allows pressure-driven infusions through specialized catheters1. However, confirmatory evidence of CED efficacy in clinics is still scarce, due to haphazard catheter positioning, unsuitable protocols, and inaccurate prediction of drug distribution. The latter may be affected by flow rate, catheter geometry, and underlying tissue microstructure2. We studied the distribution of a Gadolinium solution infused by CED in vivo by MRI, within the brain of an ovine model. Infusate distributions were measured at multiple timepoints and compared to microstructural properties as depicted by DTI, to quantitatively assess the impact of brain tissue features on drug distribution in CED.METHODS
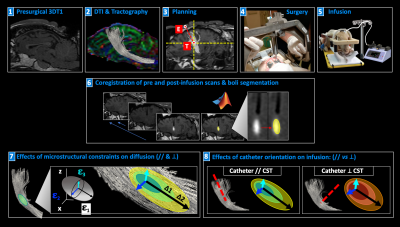
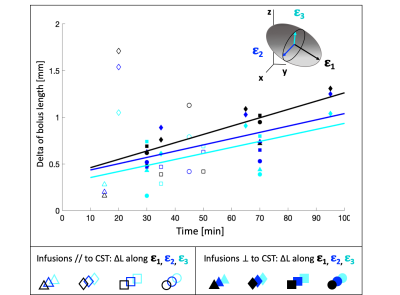
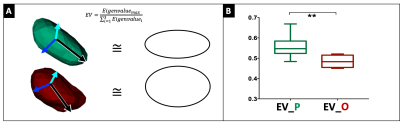
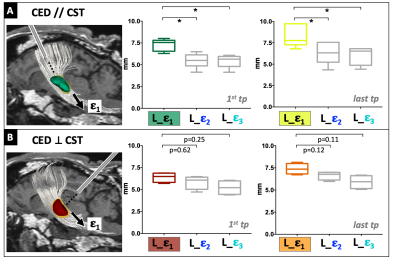
Seven sheep received a total of fourteen brain CED procedures (one per hemisphere). Fig.1 summarizes the experimental procedures and analyses. All animals underwent preoperative 1.5T MRI (Achieva, Philips Healthcare), including 3DT1 Fast-Field-Echo (FFE) (TR/TE:25/5ms) and DTI acquisitions to guide the neurosurgical planning. DTI data were collected using a single-shot echo planar sequence with parallel imaging along 15 non-collinear directions at b=1000s/mm2 (TR/TE:6700/84ms;SENSE=2), according to a previously described protocol3. After eddy-current correction by FSL, diffusion tensors and FA map were estimated and ovine corticospinal tracts (CSTs) were reconstructed using Diffusion ToolKit and TrackVis. CSTs were aligned to 3DT1-FFE images and loaded on a bespoke version of the Neuroinspire™ neurosurgical planning software (Renishaw plc.). Targeting the CST at the level of the internal capsule, the stereotactic placement of CED infusion catheters via a bespoke MRI-compatible ovine headframe and CRW stereotactic system (Figure 1.4) was parallel to CST fibers in 8 cases and orthogonal to these in 6. In each animal, 10mL of a gadolinium-based solution (Prohance®,1:80 in saline) were infused by CED at 3mL/min. Minimum one and maximum four post-infusion 3DT1-FFE consecutive scans were acquired for each sheep, from 10 to 120 minutes after CED. Pre- and post-infusion scans were aligned for each animal and normalized to a common baseline-intensity, to allow intra-sheep monitoring of infusate distribution over time. Binary masks of the infused boli were extracted by an automatic region-growing algorithm (Matlab R2019a, Mathworks), to guarantee homogeneous data handling and segmentation of gadolinium volumes across different timepoints and animals. Segmentations were approved by a board-certified neuroradiologist. A Python script was employed to compute the principal components of the boli across consecutive timepoints, and DTI-derived eigenvectors (ε1, ε2, ε3) of the underlying tissue. After obtaining bolus lengths (L) along ε1, ε2, ε3, differences in L (ΔL) between the last timepoints and the first ones allowed to analyze the elongation of infusates along each of the three DTI-directions over time. Then, shapes and elongations of gadolinium volumes were defined quantitatively by calculating the explained variance (EV) of principal components of each bolus, to compare the shape of boli in parallel or orthogonal infusions. Finally, different lengths of boli for parallel and orthogonal infusions were quantified, to evaluate the impact of catheter positioning on solution diffusion. Infusions parallel versus orthogonal to the CST were compared by means of an ANCOVA test, evaluating EV keeping time as covariate, and Wilcoxon tests, comparing L along ε1, ε2, ε3.RESULTS
Microstructural properties of the ovine brain tissue were consistently described by DTI in all animals, allowing reproducible tensor estimations and CST reconstruction with minimal inter-sheep variability. Differences in the elongation of boli (ΔL) along DTI ε1, ε2, ε3 are shown in Fig.2 for all the infusions, fitted by linear interpolations for each DTI direction. The slope of the line interpolating DL along ε1 was consistently greater than those interpolating DL along ε2 and ε3 for all infusions (Fig.2). Furthermore, comparisons between infusions delivered parallel and orthogonal to CST revealed that boli had an oval shape in both cases, but the former displayed higher EV, thus resulting significantly more elongated (Fig.3). In infusions parallel to CST fibers, the absolute L of boli along ε1 was significantly greater than L along ε2 and ε3 already at the first timepoint acquisitions, maintaining this difference over time (Fig.4A). Infusions orthogonal to fibers show a non-significant trend of a greater L along ε1, that becomes more pronounced over time (Fig.4B).DISCUSSION AND CONCLUSION
This study provides an experimental demonstration in vivo, in a large animal model, of the influence of tissue properties on drug distribution within the brain. Quantitative analyses of ΔL along ε1, ε2, ε3 over time demonstrate that microstructural constraints impact drug diffusion, facilitating it along the white matter (WM) fiber direction (ε1). Furthermore, quantitative evaluations highlight that different catheter orientations with respect to WM may influence the initial drug distribution after CED ceases, where convection effects may still play a role. Infusions parallel to white matter fibers are already driven along the fiber direction at the first timepoint, continuing to expand preferentially along ε1. This expansion is less evident in infusions orthogonal to WM, but still shows a trend along ε1.In conclusion, tissue microstructure as depicted by DTI impacted the in vivo distribution of drugs directly delivered within the brain. Knowledge of these properties is essential to plan optimal catheter positioning in individual patients and to accurately predict final drug distribution in CED.
Acknowledgements
This project has received funding from the European Union’s EU Research and Innovation programme Horizon 2020 under grant agreement no. 688279.References
1. Bobo, R. H. et al. Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci U S A 91, 2076-2080, doi:10.1073/pnas.91.6.2076 (1994).
2. Rosenbluth, K. H. et al. Analysis of a simulation algorithm for direct brain drug delivery. Neuroimage 59, 2423-2429, doi:10.1016/j.neuroimage.2011.08.107 (2012).
3. Pieri, V. et al. In vivo Diffusion Tensor Magnetic Resonance Tractography of the Sheep Brain: An Atlas of the Ovine White Matter Fiber Bundles. Frontiers in Veterinary Science 6, doi:10.3389/fvets.2019.00345 (2019).
Figures