4155
A test-retest analysis of motion compensation in image-guided radiation therapy using hands-free simultaneous MR-U/S system.1General Electric Research, Niskayuna, NY, United States, 2Medical Physics, University of Wisconsin - Madison, Madison, WI, United States, 3Radiology, University of Wisconsin - Madison, Madison, WI, United States, 4Human Oncology, University of Wisconsin - Madison, Madison, WI, United States
Synopsis
An MR-compatible ultrasound probe was developed for hands-free, simultaneous MR-ultrasound image acquisition for image-guided radiation therapy in liver. Simultaneously acquired pre-treatment MR and U/S associates a pair of MR image and U/S volume to each respiratory state over multiple respiratory cycles without requiring physical MR image acquisition during the radiation treatment LINAC phase. Automatic fiducial tracking and respiratory state clustering were performed on the U/S volumes to determine expiration states for dose delivery. In a test-retest analysis, consistent and reproducible expiration states were obtained that can be used for targeted-dose delivery under LINAC.
PURPOSE
MR provides better tissue contrast for delineating tumor margins and is used for targeted radiation dose-delivery in MR-LINAC systems. Although MR-LINAC systems1-4 can track tumor targets in real-time and can adapt to targeted dose delivery based on patient breathing, the technology is expensive for cancer treatment, and requires an entirely different system. A cost-effective alternative to MR-LINAC systems is the proposed MR-ultrasound image guided radiation therapy (IgRT) solution that is able to track tumor targets using simultaneous 4D ultrasound (U/S) and MRI5,6. The MR images acquired prior to radiation treatment under U/S guidance provides high contrast tumor visualization during active radiation treatment guidance on the LINAC.METHOD
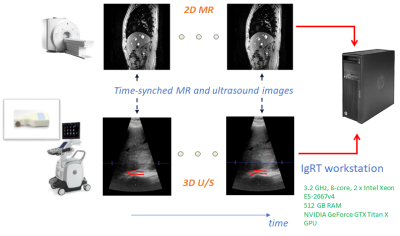
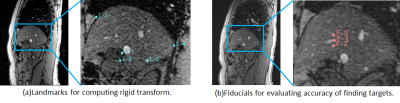
An MR-compatible 4D ultrasound probe was developed to allow hands-free, simultaneous MR-ultrasound image acquisition5,6. Simultaneously acquired pre-treatment MR and hands-free U/S associates a pair of MR image and U/S volume to each respiratory state over multiple respiratory cycles. The U/S images are used to determine the respiratory states, and the associated MR images that represent that respiratory state in real-time, without requiring physical MR image acquisition during the radiation treatment LINAC phase. To determine respiratory states, displacements of an endogenous fiducial marker, such as a blood vessel or vessel bifurcation in liver were tracked over a time sequence of U/S volumes using a fast and efficient block-matching method7. The displacements spanned the space of motion of the endogenous marker due to patient breathing and were used to automatically label the different respiratory states using a hierarchical clustering method8,9. Vector cosine distance of displacement vectors was used to derive cluster (respiratory state) labels as beam-space data in U/S do not conform to cartesian space. The cluster that occurs most often was hypothesized to signify the end of inspiration or expiration during the respiratory cycle. Because internal organs are moving least at these times, this cluster represents a good opportunity for LINAC dose delivery. All studies were conducted in a GE SIGNA MR750 or a Premier 3.0T MRI scanners with the MR-compatible ultrasound probe driven by a GE Vivid E95 ultrasound scanner. Simultaneous ultrasound and MR data were streamed to an Intel Xeon workstation (512 GB RAM and NVIDIA GeForce GTX Titan X GPU). TTL signals from the MR scanner indicating the start and end of data acquisition for each slice location in conjunction with U/S time-stamps allowed matching of MR images to 3D ultrasound volumes at each time point (Fig. 1). Both 2D MR fast gradient echo (FGRE) and 4D ultrasound images were acquired at 4 fps. Four healthy volunteers were consented under IRB-approved protocols. The U/S probe was placed on the right lateral abdominal wall and secured in place with a strap to image the liver. Two imaging sessions were conducted for the test-retest analysis in order to simulate the patient and probe re-positioning scenarios between pre-therapy and therapy procedures, i.e., the IgRT patient workflow. The fiducial tracking and clustering methods were applied independently on the two U/S volume datasets from the two sessions. Matched MR images corresponding to the end-expiration cluster labels and from the two imaging sessions were aligned using a rigid registration to evaluate the sensitivity of the tracking and respiratory clustering methods subject to patient and probe re-positioning. A total of 5 landmarks on the liver contour were used to drive the rigid registration, while 3 other fiducials within the liver were used to evaluate the accuracy of localizing the target in the expiration MR images of two independent imaging sessions.RESULTS
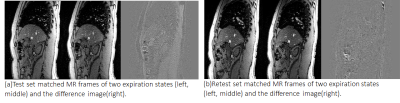
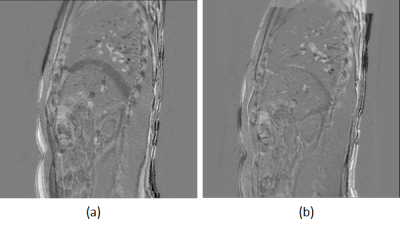
Reproducible end-expiration states were obtained automatically using the tracking and clustering methods in both imaging sessions. Fig. 2 shows the end-expiration states with cluster label ‘0’ in both the test-retest data plotted along a one-minute acquisition of U/S (~240 volumes). Fig. 3(a) and 3(b) show the difference images between the matched MR images of expiration states for test and retest data respectively for one subject. Fig. 4(a) and 4(b) shows the difference MR image between the expiration states of test and retest data before alignment and after rigid alignment respectively. Fig. 5(a) shows the fiducials used for computing the rigid transform and 5(b) shows the targets used to evaluate the target localization accuracy between expiration states. A mean value of 1.6 mm ± 1.4 mm for the Euclidean distances of 3 fiducials within the liver was achieved between the test-retest expiration states.DISCUSSIONS AND CONCLUSIONS
The clustering method consistently captured the end-expiration states in test-retest data that involved repositioning the volunteer and the probe. The error of target localization has been limited to <2 mm as required for reducing the planning treatment volume in IgRT. Further experiments will involve multiple subjects, and finally the cluster labels will be used to train a machine learning model to predict the end-respiratory state labels in real-time during treatment. The results indicate that the MR/ultrasound solution can provide the necessary image guidance for radiation treatment and can be used on any existing MR and LINAC systems, obviating the need for expensive new MR-LINAC system.Acknowledgements
Funding support: NIH R01CA190298.References
1. Park JM, Park S-Y, Kim HJ, Wu H-G, Carlson J, Kim J-I, A comparative planning study for lung SABR between tri-Co-60 magnetic resonance image guided radiation therapy system and volumetric modulated arc therapy. Radiother Oncol 2016; 120: 279-85.
2. Mutic S, Dempsey AF, The ViewRay system: magnetic resonance–guided and controlled radiotherapy, Sem Rad Oncol 2014; 24: 196-9.
3. Raaymakers BW, Lagendijk JJ, Overweg J, Kok JG, Raaijmakers AJ, Kerkhof EM, van der Put RW, Meijsing I, Crijns SP, Benedosso F, van Vulpen M, de Graaff CH, Allen J, Brown KJ, Integrating a 1.5 T MRI scanner with a 6 MV accelerator: proof of concept. Phys Med Biol 2009; 54: N229-37.
4. Kerkmeijer LG, Fuller CD, Verkooijen HM, Verheij M, Choudhury A, Harrington K, Schultz C, Sahgal A, Frank SJ, Goldwein J, Brown KJ, Minsky BD, van Vulpen M; MR-Linac Consortium Clinical Steering Committee The MRI-Linear accelerator consortium: evidence-based clinical introduction of an innovation in radiation oncology connecting researchers, methodology, data collection, quality assurance and technical development. Front Oncol, 2016, 6:215.
5. Foo TKF, et al. Proc. 26th ISMRM, p. 4416.
6. Lee W, Chan H, Chan P, Fiorillo T, Fiveland E, Foo T, Mills D, Patel A, Sabatini J, Shoudy D, Smith S, Bednarz B, A magnetic resonance compatible E4D ultrasound probe for motion management of radiation therapy. IEEE Intl Ultrasonics Symp 2017; doi: 10.1109/ultsym.2017.8092223.
7. Shepard AJ, Wang B, Foo TKF, Bednarz B, A block matching based approach with multiple simultaneous templates for the real‐time 2D ultrasound tracking of liver vessels. Med Phys 2017; 44: 5889-900.
8. Pedregosa F, Varoquaux G, Gramfort A, Michel V, Thirion B, Grisel O, Blondel M, Prettenhofer P, Weiss R, Dubourg V, Vanderplas J, Passos A, Cournapeau D, Brucher M, Perrot M, Duchesnay E, Scikit-learn: Machine Learning in Python. J Machine Learning Research 2011; 12: 2825-30.
9. Zepeda-Mendoza ML and Resendis-Antonio O. Hierarchical Agglomerative Clustering. In: Dubitzky W, et al. (eds) Encyclopedia of Systems Biology. 2013, Springer, New York, NY.
Figures