4153
Deuterium oxide as a black water contrast medium for real-time MRI-guided endovascular neurointervention1Department of Radiology and Radiological Science, The Johns Hopkins University School of Medicine, Baltimore, MD, United States, 2F.M. Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Research Institute, Baltimore, MD, United States, 3The First Affiliated Hospital of Jinan University, Guangzhou, China, 4Department of Diagnostic Radiology and Nuclear Medicine, University of Maryland, Baltimore, MD, United States
Synopsis
The use of contrast media, such as Gadolinium-based contrast agents (GBCA), superparamagnetic iron oxide particles (SPIO) and carbon dioxide, can improve the conspicuity of MRI, thereby helping to improve the accuracy of transcathether infusions during endovascular neurointerventions. In this study, we exploited deuterium oxide (D2O) as a new MRI contrast medium for guiding intra-arterial hyperosmotic blood brain barrier (BBB) opening in experimental animal models. Compared to the SPIO-based approach, D2O MRI guidance was found to provide comparable results that can predict the territory of BBB opening (assessed by Gd-MRI).
Introduction
MRI-guided endovascular intervention plays an important role in the treatment of various diseases (1-5). The use of contrast media, including Gadolinium-based contrast agents (GBCA) (6), superparamagnetic iron oxide particles (SPIO) (7) and carbon dioxide (8), can improve the conspicuity and thereby the accuracy of transcathether intra-arterial injections. However, the safety and clinical applicability of these agents are still under debate for the use in patients. Deuterium oxide (D2O) is a stable, non-radioactive isotopic form of water with similar physical and chemical properties as regular water. While traditionally D2O be only detected using MRI scanners equipped with special 2H-tuned hardware, a recent new study by Wang et al. successfully utilized the proton replacement effect of D2O to develop it as a negative contrast medium in 1H-MRI (9). In the present study, we sought to develop D2O as a novel contrast agent for image guidance during endovascular neurointervention by accurately determining the perfusion territory of intra-arterially injected solutions.Methods
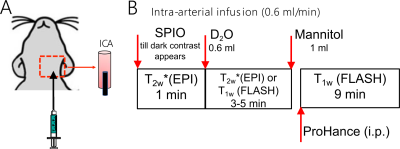
All chemicals were purchased from Sigma Aldrich (Saint Louis, MO, USA) unless otherwise stated. Animal studies were approved by institutional ACUC. Rat experiments were performed on a horizontal bore 11.7 T Bruker Biospec system (Bruker, Ettlingen, Germany), with the experimental timeline illustrated in Figure 1. The dynamic SPIO MRI were performed using an EPI sequence with TR/TE = 2s/9.7ms, segment factor = 2, slice thickness = 1 mm, FOV =3×3 cm2. The Gd-enhanced MRI were performed using a FLASH sequence with TR/TE = 100ms/1.7 ms, flip angle = 45°, average number = 8, slice thickness = 0.6 mm, and FOV =3×3 cm2. The D2O MRI was acquired either using the same EPI or FLASH sequences to compare with SPIO- or Gd- MRI, respectively. The canine experiment was performed on a clinical 3T Siemens Trio with a quadrature head coil, and the dynamic T2w* images were acquired with a GE-EPI (TE = 36 ms, TR = 3000 ms, matrix = 128, and acquisition time = 3 s and 50–100 repetitions) after the injection of SPIO or D2O. Standard Gd-enhanced images were also acquired after the injection of mannitol.Results and Discussion
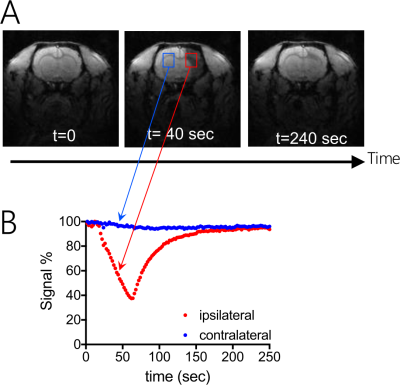
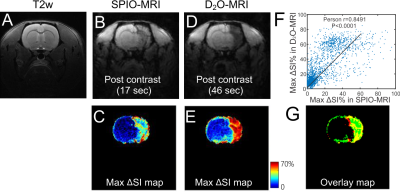
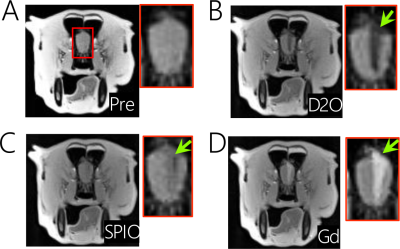
As shown in Figure 2, IA injection of 0.6 mL D2O caused a rapid (within 50 seconds) signal decrease in the ipsilateral brain hemisphere (red ROI), attributed to the proton replacement effect of locally high concentration of D2O. This decrease in MRI signal completely recovered in approximately 3 minutes due to the washout of D2O. It should be noted that in certain regions, D2O clearance took up to several minutes, indicating that D2O entered interstitial and intracellular spaces. As expected, there was almost no signal change in the contralateral hemisphere (blue ROI).Figure 3 shows the comparison between the SPIO- and D2O MRI results of a representative rat (Figures 3B and D), revealing similar contrast enhanced regions. This is further confirmed by comparing the maximal contrast-enhancement maps (Figures 3C and E) acquired by the two methods, with a good pixel-wise correlation (Pearson coefficient= 0.8491, Figure 3F) and good match in the contrast-enhanced regions, which is displayed as yellow color in the pseudocolor image (Figure 3G) composed of the maximal SPIO-enhancement (red) and maximal D2O-enhancement (green).
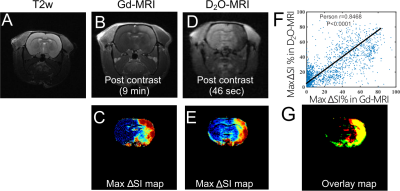
To further prove that D2O-MRI can be used to predict the transcatheter perfusion territory and BBB opening, we injected mannitol at the same flow rate as used in D2O infusion and measured the brain regions with BBB opening as assessed by Gd-enhanced MRI. The comparison of Gd-MRI and D2O-MRI is shown in Figures 4 B-E, revealing similar contrast-enhanced areas. There was a good correlation with the Gd-contrast maps, i.e., Pearson coefficient= 0.8468 (Figure 4F). The composite color image (Figure 4G) shows an outstanding match of regions from the two methods. Quantitatively, the overlapped areas account for 72.45% of the D2O-enhanced area and 73.37% of the Gd-enhanced areas, indicating that D2O-MRI can reliably predict the area of BBB opening.
Finally, we performed a 3T D2O-MRI before the interventional BBB opening for the purpose of drug delivery in a canine model. The observed infusion area of D2O (Figure 5 B) in the dog brain correlated well with that of Gd-CE (Figure 5D), and even better than that of SPIO (Figure 5C). This pilot 3T study demonstrates the feasibility of D2O-MRI on low field clinical MRI scanners.
Conclusion
Our study showed that D2O is a negative MRI contrast medium suitable for guiding endovascular neurointerventions. Using regular 1H MRI scanners, we demonstrated the ability of D2O MRI to accurately visualize intra-arterial perfusion territory and predict the regions of BBB opening by mannitol (injected using the same route) in rats and dogs. As D2O is an inexpersnive agent with well-documented safety profile, our approach is safe and cost-effective with a good quantitative ability, and may be useful for a variety of endovascular procedures.Acknowledgements
This work was supported by NIH grant R01CA211087, R01NS091110, and R21NS106436References
1.Pushparajah K, Chubb H, Razavi R. MR-guided Cardiac Interventions. Top Magn Reson Imaging 2018;27(3):115-128.
2. Saeed M, Saloner D, Weber O, Martin A, Henk C, Higgins C. MRI in guiding and assessing intramyocardial therapy. Eur Radiol 2005;15(5):851-863.
3. Ozturk C, Guttman M, McVeigh ER, Lederman RJ. Magnetic resonance imaging-guided vascular interventions. Top Magn Reson Imaging 2005;16(5):369-381.
4. Lillaney PV, Yang JK, Losey AD, Martin AJ, Cooke DL, Thorne BR, Barry DC, Chu A, Stillson C, Do L, Arenson RL, Saeed M, Wilson MW, Hetts SW. Endovascular MR-guided Renal Embolization by Using a Magnetically Assisted Remote-controlled Catheter System. Radiology 2016;281(1):219-228.
5. Janowski M, Walczak P, Pearl MS. Predicting and optimizing the territory of blood-brain barrier opening by superselective intra-arterial cerebral infusion under dynamic susceptibility contrast MRI guidance. J Cereb Blood Flow Metab 2016;36(3):569-575.
6. Frayne R, Omary RA, Unal O, Strother CM. Determination of optimal injection parameters for intraarterial gadolinium-enhanced MR angiography. J Vasc Interv Radiol 2000;11(10):1277-1284.
7. Wacker FK, Reither K, Ebert W, Wendt M, Lewin JS, Wolf K-J. MR Image–guided Endovascular Procedures with the Ultrasmall Superparamagnetic Iron Oxide SH U 555 C as an Intravascular Contrast Agent: Study in Pigs. Radiology 2003;226(2):459-464.
8. Wacker FK, Maes RM, Jesberger JA, Nour SG, Duerk JL, Lewin JS. MR Imaging–Guided Vascular Procedures Using CO2as a Contrast Agent. American Journal of Roentgenology 2003;181(2):485-489.
9. Wang FN, Peng SL, Lu CT, Peng HH, Yeh TC. Water signal attenuation by D2O infusion as a novel contrast mechanism for 1H perfusion MRI. NMR Biomed 2013;26(6):692-698.
Figures