4152
Development of an optimal head localizer for stereotactic neurosurgery at 7.0T with minimal image geometric distortions1Department of Neurosurgery, Mayo Clinic, Rochester, MN, United States, 2Medical Scientist Training Program, Mayo Clinic, Rochester, MN, United States, 3Department of Radiology, Mayo Clinic, Rochester, MN, United States, 4Department of Engineering, Mayo Clinic, Rochester, MN, United States, 5Department of Physiology and Biomedical Engineering, Mayo Clinic, Rochester, MN, United States
Synopsis
7.0T MRI provides precise visualization and targeting of brain structures for image-guided stereotactic neurosurgery. However, image localizers used by stereotactic systems do not exist for 7.0T scanners. Challenges in their development include: the small bore creates geometric constraints that disallow use of conventional localizers, and the increased B0 increases geometric distortion, affecting registration accuracy. Here, a skull-contoured localizer utilizing point fiducials was designed to attach to a novel stereotactic frame. Extracranial distortion was thoroughly mapped using several optimized imaging sequences. This data was used to optimally place fiducials on the localizer, improving registration accuracy.
Introduction
MRI is critical to functional neurosurgery for navigation and accurate targeting of specific neurologic structures and pathologies.1 This is especially important in the approach for deep brain stimulation (DBS).2 3.0T MRI does not afford the necessary signal/contrast-noise ratios (SNR/CNR) to resolve small neural targets, and hence indirect targeting methods (human brain atlases) and intra-operative microelectrode recordings (target verification) are required for placement of DBS electrodes. 7.0T MRI offers the potential to parcellate brain structures for direct targeting and identify unseen neural connections through diffusion tensor imaging.3 Thus, 7.0T MRI offers safer, more effective, and more individualized surgery.For high quality 7.0T imaging to be applicable to stereotactic targeting, the stereotactic image localizer must fit within the narrow head coil. Presently, conventional N-bar designs are much too large. Another major problem is the increased geometric distortion with 7.0T which, for stereotactic surgery, can lead to inaccuracies in intervention and sub-optimal outcomes. Image geometric distortions cause shifts in true fiducial locations which impacts registration and ultimately targeting accuracy.4 Sources of distortion include gradient nonlinearities, imperfect B0 shimming, B1 inhomogeneity, chemical shift artifacts, and susceptibility-induced distortions. Optimal sequence design can mitigate these distortions, while maintaining the required image spatial and contrast resolution for target visualization.
The aim of this study was to design a head localizer frame with fiducial placements at extracranial locations exhibiting minimal geometric distortions when imaged with optimized imaging sequences at 7.0T. Sequences were refined in phantom and human experiments, with extracranial distortions thoroughly mapped with high spatial resolution in the 3D space.
Methods
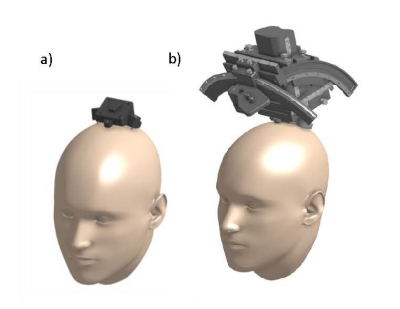
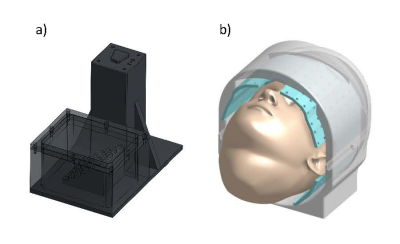
A prototype skull-contoured localizer was designed to fit within the reduced diameter of the FDA-approved head coil (1Tx/32Rx, Nova Medical). The localizer integrated with a novel “D1” stereotactic system previously developed in our laboratory (Figure 1). This system utilizes a small skull-secured device platform that permits rigid attachment of the image localizer to the skull. The localizer had 8 custom-made 4mm spherical fiducials placed in a unique geometric pattern on the localizer to allow for proper right/left, anterior/posterior, and superior/inferior image registration. The localizer was affixed to imaging phantoms with targetable points and filled with oil (Marcol 82); this set-up was used to determine fiducial and target registration errors (FRE/TRE), and for protocol optimization (Figure 2a). Human imaging (n=3) was performed with approval from the local IRB (IRB#19-002599), at 7.0T (Terra, Siemens). MPRAGE, FGATIR, T2-w, and SWI imaging sequences were optimized (Table 1). The receiver bandwidth was varied, commensurate with adequate SNR, to minimize geometric distortions. A trained neurosurgeon evaluated the images to ensure good visualization of the target nuclei (subthalamic nucleus, globus pallidus, ventrointermedial thalamus).Extracranial distortion was analyzed via a custom distortion analysis device that fit around the subject’s head within the head coil, comprising 46 fiducial locations with known ‘ground truth’ positions (Figure 2b). Following rigid image registration, the global and local distortions were determined for each image sequence using custom python software. This was used to refine the initial prototype design of the localizer for fiducial placement in areas of low distortion. The finalized optimized localizer was used in phantom studies to investigate the improvement in registration errors (both TRE and FRE).
Results
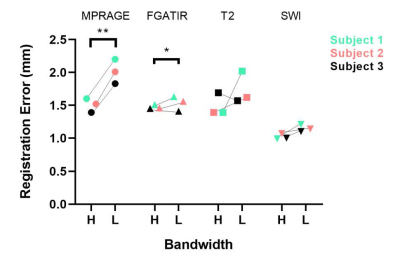
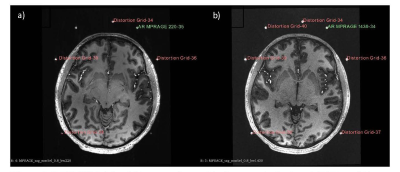
Phantom testing with the prototype localizer revealed an FRE/TRE of 3.69-6.43mm/ >10mm, respectively. Initial parameterization revealed that increased receiver bandwidth significantly reduced this degree of error.Human tests were then conducted using the distortion analysis device. Low bandwidth sequences demonstrated an overall higher geometric distortion (FRE: 1.61mm) compared to high bandwidth sequences (FRE: 1.37mm) (n=3 for each unique sequence). For high bandwidth sequences, registration errors of 1.5±0.09mm, 1.49±0.14mm, 1.47±0.03mm, and 1.02±0.04mm were found for MPRAGE, FGATIR, T2, and SWI, respectively. For low bandwidth sequences, registration errors of 2.01±0.15mm, 1.74±0.2mm, 1.53±0.09mm, and 1.15±0.05mm were found for MPRAGE, FGATIR, T2, and SWI, respectively. Statistically significant increases in fiducial registration error were found between high and low bandwidths in MPRAGE and FGATIR sequences (paired t-test, Figure 3, 4).
In addition, individual displacement of fiducials after registration compared with their known ground truth locations permitted the creation of 3D distortion maps. These maps were used to modify the image localizer for placement of fiducials in areas that demonstrated reduced distortion (image not shown, patent pending). Overall, this new localizer demonstrated a FRE of 1.2-1.4mm and a TRE of 0.72-2.4mm.
Discussion
7.0T imaging enhanced visualization of neural targets. Detailed mapping of extracranial distortion among a number of clinical sequences demonstrated nonuniform magnitudes of distortion, with lower distortion for the high bandwidth sequences. Relatively increased distortion was observed at the base of the skull and above the apex of the head, with lower distortion near the center of the head. These data helped guide the precise fiducial placement on the finalized localizer design, in extracranial locations that demonstrated the least amount of distortion. The registration errors (1-2mm) found with this new localizer are in the clinically acceptable range and will be used in further experiments to determine its accuracy for stereotactic targeting. Furthermore, the distortion data generated here is generalizable to other applications, such as placement of skin fiducials or eventual 7.0T intra-MRI surgery.Acknowledgements
We would like to acknowledge the Mayo Clinic Department of Radiology for their support of our imaging experiments. We would also like to acknowledge our sources of support: the Grainger Foundation, NIH MSTP T32 (GM065841) and NRSA TL1 (TR002380) training grants.References
1. Kelly, P. J., Sharbrough, F. W., Kall, B. A. & Goerss, S. J. Magnetic resonance imaging-based computer-assisted stereotactic resection of the hippocampus and amygdala in patients with temporal lobe epilepsy. Mayo Clin Proc 62, 103-108, doi:10.1016/s0025-6196(12)61877-1 (1987).
2. Cho, Z. H. et al. Direct visualization of deep brain stimulation targets in Parkinson disease with the use of 7-tesla magnetic resonance imaging. J Neurosurg 113, 639-647, doi:10.3171/2010.3.JNS091385 (2010).
3. Lenglet, C. et al. Comprehensive in vivo mapping of the human basal ganglia and thalamic connectome in individuals using 7T MRI. PLoS One 7, e29153, doi:10.1371/journal.pone.0029153 (2012).
4. Voormolen et al. Implications of Extracranial Distortion in Ultra-High-Field Magnetic Resonance Imaging for Image-Guided Cranial Neurosurgery. World Neurosurgery 26:e250-e258, doi: 0.1016/j.wneu.2019.02.028
Figures