4151
Towards Quantitative Myocardial Perfusion: Catheter-Based Arterial Input Function Measurements with Flow-Normalization1Dept. of Radiology, Medical Physics, Medical Center University of Freiburg, Faculty of Medicine, University of Freiburg, Freiburg, Germany, Freiburg, Germany
Synopsis
For quantitative myocardial perfusion measurements with intra-arterial contrast agent (CA) injection precise knowledge of the arterial input function (AIF) is needed. Not necessarily absolute values of the AIF are needed if blood flow measurements are used to normalize the perfusion maps. In this work it is shown for a constant-flow phantom that this method works and probable issues for transferring the method to in-vivo measurements are discussed.
Introduction
In MR-guided coronary catheterizations contrast agent (CA) is directly injected through a catheter, and the dynamic signal change in the myocardium is mapped to assess myocardial perfusion defects and vascular supply territories1,2,3. Recently it was shown that it is also possible to use the tracking coil of an active catheter to determine the arterial input function (AIF) for quantitative myocardial perfusion measurements4. In this work a deconvolution analysis5 was performed to create quantitative perfusion maps. Systematic differences in the AIF determination were seen between AIFs from active tracking coils and input functions that are measured in large blood vessels4. To overcome this limitation, in this work we developed a normalization method which is using the measured blood flow around the catheter coil to normalize the perfusion values.Materials and Methods
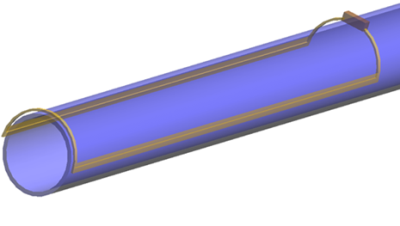
To mimic arterial blood flow into myocardial tissue a perfusion phantom was constructed from a plastic tube which was connected in series to a dialysis filter. An active catheter (8F, see fig. 1) was inserted into the upstream section of the tube to inject CA and measure the AIF. MR imaging experiments were performed at a clinical 3T MRI system (Prisma, Siemens) using a FLASH sequence with saturation recovery preparation. The dynamic signal change was imaged before, during and after CA injection (10ml-bolus, 0.5% Gd-DTPA) via the catheter. The following imaging parameters were used: TE=1.6ms, TR=4.0ms, α=8°, (Δx)3=0.8×0.8×8.0 mm3. The image plane was oriented such that it encompassed both the dialysis filter and the supplying tube to be able to determine a reference AIF from the images. The catheter AIF was measured by sampling data in an additional acquisition block without spatial encoding only from the catheter coil.Before and after the perfusion measurement the volume flow rate of the supplying tube was determined using a phase contrast flow measurement (VENC=60cm/s). The measured volume flow rate was used for the flow correction of the measured AIF using the relation4,6: $$$[Gd]_\mathrm{Blood}=\frac{1}{1+q}[Gd]_\mathrm{inj}$$$ with the volume flow rate ratio $$$q=\frac{Q_\mathrm{Blood}}{Q_\mathrm{inj}}$$$.
A deconvolution analysis5 was implemented to extract quantitative perfusion maps from the AIF. For every voxel the measured concentration-time curve of the myocardium was fitted by a convolution of the AIF and a Fermi function. The perfusion map was normalized by spatial integration and normalization to the flow measured in the supplying tube, because the absolute concentration of the CA in the AIF was not known.
Results
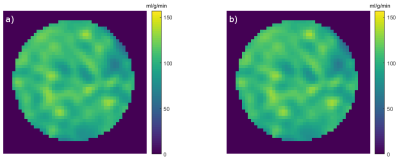
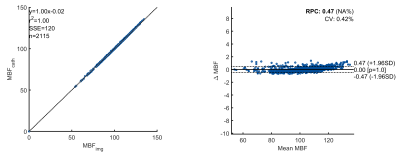
The perfusion maps shown in fig. 2 show that the perfusion map of the dialysis filter is almost identical for the image-based and the catheter-based method. A quantitative comparison between the two methods shows an excellent linear correlation (r² = 1.0) and no systematic flow-dependent deviations (Bland-Altman plots, fig. 3).Discussion & Conclusion
The results show the possibility of measuring quantitative perfusion in the perfusion phantom without the knowledge of the absolute AIF. Since the perfusion phantom represents an ideal situation, not all relevant features of the myocardium are represented, and the results cannot be transferred exactly to in-vivo measurements. Firstly, the perfusion phantom provides a constant flow, whereas the myocardial blood flow is pulsatile. This limitation might be overcome using time-resolved flow measurements and averaging over the cardiac cycle, which can easily be achieved using fast projection measurements from the tip tracking coil7.In conclusion this work shows that the precision of perfusion measurements with AIFs from catheter coils might be substantially increased by adding flow information around the catheter coil. The technique has shown excellent results in a phantom setup and will be further tested in in-vivo measurements.
Acknowledgements
This work was partially supported by an institutional cooperation with Siemens. Support from A. Hengerer, F. Maier and A. Krafft (Siemens) is gratefully acknowledgedReferences
1. von Zur Mühlen C, Reiss S, et al. Coronary magnetic resonance imaging after routine implantation of bioresorbable vascular scaffolds allows non-invasive evaluation of vascular patency. PLoS One 2018; 25:13(1)
2. Spuentrup E, Ruebben A, Schaeffter T, et al. Magnetic Resonance–Guided Coronary Artery Stent Placement in a Swine Model. Circulation 2002;105(7):874–879
3. Omary RA., Green JD, Schirf BE. Real-Time Magnetic Resonance Imaging-Guided Coronary Catheterization in Swine. Circulation 2003;107(21):2656–2659.
4. Stephan S, et al. Catheter-based Arterial Input Function for Quantitative Perfusion Measurements with Intra-arterial Injection. Proc. Intl. Soc. Mag. Reson. Med. 27; 2019
5. Wilke N, Jerosch-Herold M, et al. Myocardial Perfusion Reserve: Assesment with Multisection, Quantitative, First-Pass MR Imaging. Cardiac Radiology 1997;204:373-384
6. R. Frayne, R. A. Omary, et al. Determination of Optimal Injection Parameters for Intrarterial Gadolinium-enhanced MR Angiography. Journal of Vascular and Interventional Radiology 2000;11:1277-1284
7. Volz S, et al. Semiquantitative fast flow velocity measurements using catheter coils with a limited sensitivity profile. Magn. Reson. Med. 2004; 52: 575-581