4150
Mixed reality neuronavigation for transcranial magnetic stimulation treatment1Radiology, Stanford University, Stanford, CA, United States, 2Psychiatry, Stanford University, Stanford, CA, United States, 3MIRECC, VA Palo Alto Health Care System, Palo Alto, CA, United States, 4Neuroscience, Massachusetts General Hospital, Charlestown, MA, United States, 5Radiology, Harvard Medical School, Charlestown, MA, United States
Synopsis
Transcranial magnetic stimulation (TMS) treatment is an important non-invasive treatment option for depressive patients who do not respond to medication. Effective TMS treatment is dependent on accurate stimulation intensity calibration and coil positioning, which is best achieved through image-based neuronavigation. Unfortunately, most TMS treatments use generalized scalp measurements, which are faster and cheaper but less accurate and less effective than image-based neuronavigation. We present a mixed reality neuronavigation system that allows the clinician to view brain MRI and targeting information superimposed on the patient’s head for an accelerated and simplified procedure
Introduction
TMS aims to modulate specific brain regions responsible for mood and behavior. A coil close to the skull produces magnetic fields that induce electric fields in the adjacent brain tissue, triggering action potentials [1]. Repetitive TMS (rTMS) applies trains of stimulation pulses and when focused on the dorsolateral prefrontal cortex (DLPFC), has been shown to alleviate depression symptoms providing complete remittance in 38% and 34% of depression patients respectively [2]. Since rTMS therapy targets specific brain circuits and requires repeated application (5 times/week for 4-6 weeks), its efficacy is highly dependent on consistent, accurate coil positioning [3]. We present a mixed reality neuronavigation system that projects all targeting information directly in the field of view of the clinician using the MagicLeap One (Magic Leap, Plantation, Florida) heads-up augmented reality system. The mixed reality neuronavigation system includes visualization of the targeting information based on the patient’s individual MRI data, alignment of the MRI data to the patient’s head, tracking of the patient’s head and tracking of the TMS coil.Methods
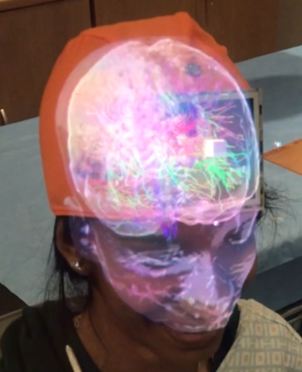
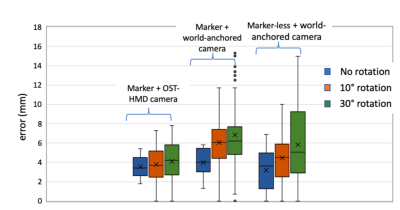
For brain surface visualization and patient head alignment we performed a 3D BRAVO brain scan on a GE 3T Premier MRI scanner with a GE 8 channel head coil. A diffusion MRI scan (single-shot EPI, 80+1 directions, b=1000 s/mm2, TE/TR = 60/8000, 2mm iso) and an angiography scan (3D Circle of Willis Time of Flight, TE/TR 2.3/21, 0.47x0.47x0.6mm) were acquired for visualization of the vasculature. A functional MRI scan (spiral, TE/TR 30/2000, 1.7x1.7x3mm) was acquired with an interleaved finger tapping task to measure the location of the hand knob area in the motor cortex. The FIND atlas was co-registered to the 3D BRAVO scan to estimate network node locations. The head and brain surfaces, the diffusion tracts, the vasculature and the functional activation in the motor area were converted to mesh surfaces and rendered on the Magic Leap One device using the Unity 3D game engine (Unity Technologies, San Francisco, USA). The head MRI rendering was co-registered to the subject’s head via fiducials placed at anatomical landmarks so that virtual rendering and real head were perceived to be accurately aligned by the operator. For head tracking, we tested 3 marker-based and marker-less tracking methods. Two marker-based approaches with an image marker attached to the head and tracked with the headset camera and an external camera and a marker-less approach where the head pose was estimated with an external depth camera [4]. The coil was tracked with an image marker, which was tracked by the MagicLeap camera. The distance of the coil center to the handknob area as well as to each of the rsfMRI networks was constantly measured. The motor threshold was estimated on the same volunteer with mixed reality neuronavigation, without navigation and with the commercial visor2 neuronavigation system.Results
A photograph of the setup is shown in Figure 1 with the TMS operator wearing the MagicLeap device and aiming at the handknob area in the motor cortex. Figures 2 & 3 demonstrate some of the functionality of this neuronavigation system with Figure 2 showing the brain surface and diffusion fiber tracts projected on the subject’s head and Figure 3 showing the functional network closest to the current TMS coil position. Figure 4 displays a plot of the accuracy measurements for the different head tracking methods. The motor threshold of the volunteer measured with all three methods was 65% without neuronavigation, 60% with the AR neuronavigation and 60% with the visor2 neuronavigation system.Discussion
We have presented a complete setup of a mixed reality neuronavigation system including rendering of the patient’s brain surface, fiber tracts, vasculature, functional areas, TMS coil tracking and patient head tracking. Initial stimulation experiments on a volunteer inspire confidence that the mixed reality neuronavigation system enables accurate localization of brain regions such as the handknob area in the motor cortex. A more extensive study with a larger cohort is underway to evaluate the consistency, setup time and ease-of-use of the mixed reality neuronavigation system compared to no neuronavigation or a commercial neuronavigation system. Ultimately, we aim to enable widespread use of image-based TMS neuronavigation through the use of a mixed-reality environment and thereby improve treatment outcomes, minimize risk and lay the groundwork for future TMS therapiesAcknowledgements
This work was supported by the grants NIH/NIMH R21 MH116484; NIH/NINDS R01 NS095985; NIH/NIMH R01 MH111444 and the Charles A. Dana Foundation.References
[1] Hallett, M., 2000. Transcranial magnetic stimulation and the human brain.
Nature 406, 147–150.
[2] Berlim, M.T., Van den Eynde, F., Daskalakis, Z.J., 2013. Clinically meaningful efficacy and acceptability of low-frequency repetitive transcranial magnetic stimulation (rTMS) for treating primary major depression: a meta-analysis of randomized, double-blind and sham-controlled trials. Neuropsychopharmacology 38, 543–551.
[3] Fitzgerald, P.B., Hoy, K., McQueen, S., Maller, J.J., Herring, S., Segrave, R., Bailey, M., Been, G., Kulkarni, J., Daskalakis, Z.J., 2009. A Randomized Trial of rTMS Targeted with MRI Based Neuro-Navigation in Treatment-Resistant Depression. Neuropsychopharmacology 34, 1255–1262
[4] Ren, C.Y., Prisacariu, V., Murray, D., Reid, I., 2013. STAR3D: Simultaneous tracking and reconstruction of 3D objects using RGB-D data. Proc. IEEE Int. Conf. Comput. Vis. 1561–1568.
Figures