4149
MRI-based motion characterisation of atrial fibrillation cardiac radiosurgery targets.1ACRF ImageX Institute, University of Sydney, Sydney, Australia, 2Auckland City Hospital, Auckland, New Zealand, 3University of Auckland, Auckland, New Zealand
Synopsis
Cardiac radiosurgery for atrial fibrillation (AF) is challenging; the target moves with both cardiac contraction and respiration and is in close proximity to critical structures. This study utilized non-contrast MRI to characterize AF cardiac radiosurgery target motion as well as relative target displacement to surrounding structures. The absolute and relative target motion seen in this study in combination with target proximity to the aorta and esophagus highlights the importance of carefully selecting cardiac radiosurgery technology and techniques. This MRI-based methodology could be useful in the AF cardiac radiosurgery clinical workflow to optimize treatment for the individual.
Introduction
Cardiac radiosurgery is an emerging non-invasive treatment alternative for Atrial Fibrillation (AF)1,2. It utilizes external beam radiation to induce circumferential myocardial scarring around pulmonary vein antra, analogous to catheter ablation induced fibrosis. Cardiac radiosurgery is challenged by target motion with cardiac contraction and respiration, variability of heart rates and atrial filling, and close target proximity to critical structures such as the esophagus and aorta.Animal and human studies studying atrial and pulmonary vein motion with respiration3,4 and/or cardiac contraction5–9 have highlighted the complexity of motion of structures within the atria. Further motion characterization of the specific AF cardiac radiosurgery treatment target is required to guide treatment technique and technology choice. No study to date has specifically assessed AF cardiac radiosurgery target motion with MRI combined with relative target displacement to surrounding structures. This study utilizes MRI to measure absolute and relative AF cardiac radiosurgery target motion in humans due to cardiac contraction and respiration.
Method
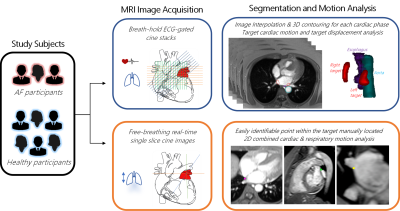
An overview of the study methodology is illustrated in Figure 1.Study subjects: Three patients diagnosed with AF and scheduled to undergo catheter ablation (1 female, 65 ± 4 years) and six healthy volunteers with no known cardiac conditions (3 female, 46 ± 15 years) were recruited into this study. Ethical approval was obtained from New Zealand Health and Disability Ethics Committee.
MRI Image Acquisition: Non-contrast cardiac MRI images were acquired on a 3T system (Siemens MAGNETOM Skyra). (i) To analyse target motion due to cardiac contraction and relative target motion to surrounding structures, ECG-triggered FLASH 2D cine images were acquired under breath-hold (TE = 2.65ms, TR = 22-28ms, FA = 10°, resolution = 1.5-2mm x1.5-2mm x 5mm, number of cardiac phases = 24-25). 8-16 contiguous slices were acquired in transverse, coronal, and sagittal planes to encompass the atria and pulmonary vein antra. (ii) To analyse target displacement due to combined respiratory and cardiac motion, free-breathing real-time True-FISP single slice cine images were acquired in three orthogonal planes for a 30-90 second period to capture multiple respiration cycles (TE = 2.5-2.6ms, TR = 201-207ms, resolution = 1.5-2mm x 1.5-2mm x 5mm, 240-250ms temporal resolution).
Segmentation and Motion Analysis: (i) Cine stacks were interpolated into static images of 1mm3 voxel size for each cardiac phase. The targets, esophagus, and aorta were segmented to create 3D contours for each cardiac phase. Target contours were of approximately 3-4mm width and depth, encompassing myocardium and pulmonary vein transmural at the pulmonary vein antra. The displacement of the centroid of the targets throughout the cardiac cycle was measured. This was all performed in MIM Maestro (MIM Software Inc, USA). The minimum relative displacement of the targets to the esophagus and aorta were measured in systole and diastole using MATLAB (Mathworks, USA). (ii) Maximum target displacement due to combined respiratory and cardiac motion was measured in MIM Maestro by manually locating an easily identifiable point within the target volume on every frame of the free-breathing cine images.
Results
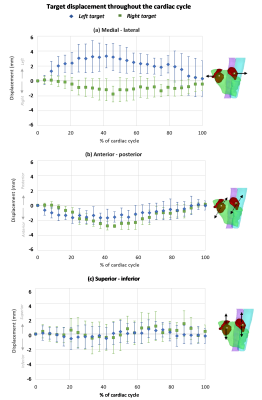
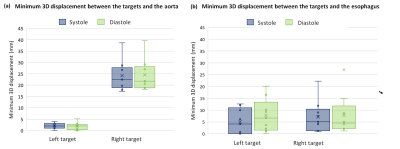
The maximum 3D displacement of the left target due to cardiac contraction was 5.0±1.0mm and 5.4±1.6mm in healthy and AF participants respectively. The maximum displacement of the right target was 4.7±0.9mm and 3.6±0.3mm in healthy and AF participants respectively. Motion was largest in the medial-lateral direction with the left and right targets displacing laterally relative to each other (Fig 2a). Both targets moved anteriorly during diastole (Fig 2b) and had minimal superior-inferior motion (Fig 2c) with cardiac contraction. The aorta was within 5mm of the left target in all participants (Fig 3a). The esophagus was in direct contact with the left target in 3 study participants (Fig 3b). Average target volume displacement on free-breathing images was 5.1±1mm, 4.9±1mm, and 11±2mm in the medial-lateral direction, anterior-posterior and superior-inferior directions respectively.Discussion
The differing lateral displacement of the left and right targets indicates that cardiac motion compensation should be optimized for each target. The combination of target motion magnitude with cardiac contraction (3.6-5.4mm) and the close proximity of the aorta to the left target volume (<5mm) informs the requirements of treatment delivery and motion compensation precision. The variable proximity of the esophagus to the targets between participants suggests that anatomical and motion analysis of potential treatment candidates may be required as part of the patient selection process. Target motion with respiration highlights the vitalness of respiratory motion compensation. Participant recruitment is on-going to further assess the variability between AF participants.This study has illustrated the suitability of MRI to comprehensively evaluate AF cardiac radiosurgery target motion. This proposed MRI-based methodology for motion characterization could be utilized within the AF cardiac radiosurgery clinical workflow to optimize treatment techniques for the individual or allow an MRI-guided clinical workflow without the requirement of alternate imaging modalities.
Conclusion
MRI was used to characterize AF cardiac radiosurgery target motion. The magnitude of target motion, the proximity of the targets to the aorta and esophagus, and the differing lateral motion in the left and right targets highlights the importance of carefully selecting cardiac radiosurgery technology and techniques. The proposed MRI-based target motion characterization methodology could be useful within the clinical workflow of AF cardiac radiosurgery.Acknowledgements
This work was funded by an Auckland Academic Health Alliance (AAHA) project grant.References
1. Cuculich PS, Schill MR, Kashani R, et al. Noninvasive cardiac radiation for ablation of ventricular tachycardia. N Engl J Med. 2017;377(24):2325-2336. doi:10.1056/NEJMoa1613773
2. Refaat MM, Zakka P, Youssef B, Zeidan YH, Geara F, Al-Ahmad A. Noninvasive Cardioablation. Card Electrophysiol Clin. 2019;11(3):481-485. doi:10.1016/j.ccep.2019.05.008
3. Ector J, De Buck S, Loeckx D, et al. Changes in left atrial anatomy due to respiration: Impact on three-dimensional image integration during atrial fibrillation ablation. J Cardiovasc Electrophysiol. 2008;19(8):828-834. doi:10.1111/j.1540-8167.2008.01128.x
4. Ipsen S, Blanck O, Oborn B, et al. Radiotherapy beyond cancer: target localization in real-time MRI and treatment planning for cardiac radiosurgery. Med Phys. 2014;41(12):120702. doi:10.1118/1.4901414
5. Bahig H, de Guise J, Vu T, et al. Analysis of Pulmonary Vein Antrums Motion with Cardiac Contraction Using Dual-Source Computed Tomography. Cureus. 2016;8(7):e712. doi:10.7759/cureus.712
6. Hasnain A, Suzuki A, Wang S, et al. Quantitative assessment of cardiac motion using multiphase computed tomography imaging with application to cardiac ablation therapy. In: SPIE-Intl Soc Optical Eng; 2018:76. doi:10.1117/12.2295438
7. Thiagalingam A, Reddy VY, Cury RC, et al. Pulmonary vein contraction: Characterization of dynamic changes in pulmonary vein morphology using multiphase multislice computed tomography scanning. Hear Rhythm. 2008;5(12):1645-1650. doi:10.1016/j.hrthm.2008.09.010
8. Rettmann ME, Holmes DR, Johnson SB, Lehmann HI, Robb RA, Packer DL. Analysis of left atrial respiratory and cardiac motion for cardiac ablation therapy. In: Medical Imaging 2015: Image-Guided Procedures, Robotic Interventions, and Modeling. Vol 9415. SPIE; 2015:94152L. doi:10.1117/12.2081209
9. Patel AR, Fatemi O, Norton PT, et al. Cardiac cycle-dependent left atrial dynamics: implications for catheter ablation of atrial fibrillation. Hear Rhythm. 2008;5(6):787-793. doi:10.1016/j.hrthm.2008.03.003
Figures