4147
Development of a first principles-based model of brain thermoregulation and initial validation with MR chemical shift thermometry1Department of Biomedical Engineering, Georgia Institute of Technology and Emory University, Atlanta, GA, United States, 2Woodruff School of Mechanical Engineering, Georgia Institute of Technology, Atlanta, GA, United States, 3Department of Radiology and Imaging Sciences, Emory University School of Medicine, Atlanta, GA, United States, 4Department of Neurology, Emory University School of Medicine, Atlanta, GA, United States, 5Petit Institute for Bioengineering and Bioscience, Georgia Institute of Technology, Atlanta, GA, United States
Synopsis
Brain temperature regulation is a key parameter after injury and ischemia. Experimental magnetic resonance (MR)-based thermometry is promising but not always clinically feasible. To complement MR thermometry measures, an improved thermal model of the brain based on first principles has been developed that rigorously ensures energy conservation in thermally interacting brain domains. As initial validation, a temperature map was generated using our model with MR angiography (MRA) and structural MR imaging (MRI) data, and compared with experimental chemical shift thermometry (CST) in the same subject. Biophysical models provide further insight into brain thermoregulation by complementing experimental thermometry measurements.
Introduction
Brain temperature is an important parameter after injury and ischemia, and increased temperature is associated with worse patient outcomes. Cerebral thermoregulation is poorly understood due to the complicated physiology of thermal homeostasis1 and a lack of non-invasive, clinically integrated thermometry tools. MR-based methods such as CST are promising; however, clinical use is limited. For example, MR thermometry immediately after ischemia is rare and continuous thermometry during thermal therapies is often not feasible. As a complement to the development of experimental brain thermometry methods, biophysical modeling can facilitate further understanding and predictions of brain temperature in both healthy subjects and patients. Building upon the recently developed VaPor approach,2 we have modified the formulation to ensure local conservation of energy and mass using the first principles of underlying thermal physics and mass-energy transport. To assess the validity of our approach, we compared the results from our augmented brain temperature model with experimentally measured temperatures using whole-brain CST.Methods
MR data was collected from a healthy male subject (31 years old) on a 3T MR scanner (PrismaFit, Siemens, Erlangan, Germany) using a 32-channel phased array head coil (Siemens). A T1-weighted magnetization-prepared rapid gradient-echo (MPRAGE) sequence (TR/TI/TE=2300/900/3.39ms, flip angle=9°, FOV=256mm×256mm, matrix size=192×192, 160 slices, slice thickness=1 mm) was used to acquire high resolution structural images. MRA was collected using a 3D time-of-flight (TOF) sequence (TR/TE/flip angle=22ms/3.86ms/15°). Echo-planar spectroscopic imaging (EPSI) (TR1/TR2/TE= 1551/511/17.6ms, flip angle=71°, FOV=280mmx280mm, and interpolated resolution=64x64x32) was acquired for MR CST. The VaPor model inputs publicly available MRI data2 to create a generalized temperature map. Here, we used individual MR data as the input to our augmented biophysical brain thermal model to facilitate the generation of subject-specific brain temperature maps. 1D arterial and venous structures within a 3D porous tissue structure form the simulation domains for solving the bioheat equations that account for metabolic heat generation balanced by heat conduction (within the solid domain), convection (between the flowing blood and tissue), and advection (within the major blood vessels and capillaries) and are used to generate brain temperature maps. Energy conservation was ensured in both the formulation of governing equations and the numerical discretization scheme used for numerically solving both the mass and momentum conservation equations. A subject-specific temperature map was generated using experimentally collected MRI and MRA data. T1-weighted images were segmented into six domains (gray matter, white matter, cerebrospinal fluid, soft tissue, skull, and background mask) using SPM 12.3 Arterial structure was constructed from 3D TOF-MRA images. A stack of these images was reconstructed into a 3D maximal intensity projection structure for vessel segmentation using the semi-automatic neuron morphing tool, neuTube 1.0,4 to identify locations of vessel nodes, diameters, and connections. Venous structure was constructed from publicly available venogram data.2 Vessel structure was augmented using a randomly exploring random tree (RRT) algorithm.5 A predicted temperature map was then generated by applying the brain thermal model with this subject-specific input data and compared to experimental CST-derived temperatures. Whole-brain temperature maps were generated from EPSI data using MIDAS6,7 and the chemical shift difference between water and N-acetylaspartate.8Results
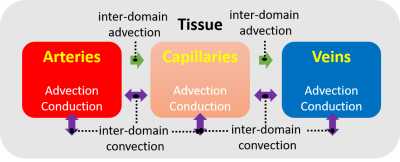
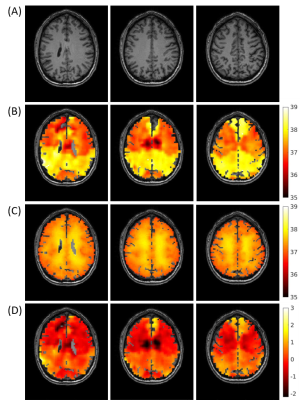
Figure 1 defines the modes of heat transfer in the brain that are included in the biophysical model of the underlying hemodynamics and thermal energy transport. A schematic for the subject-specific brain temperature modeling is shown in Figure 2. The outermost boundary temperature was 33.5°C, approximated from human scalp temperature under ambient conditions.9 Figure 3 shows the axial, coronal, and sagittal views of the subject-specific temperature map simulated by the model. Initial model validation was performed by comparing the simulated subject-specific temperature maps with experimental brain temperature maps using CST (Figure 4). Mean differences between the three representative slice pairs of simulated and experimental brain temperatures were 0.01°C, 0.02°C, and 0.27°C left to right, respectively.Discussion
Initial validation of the brain thermal model demonstrates a promising agreement between the model-predicted and experimentally measured brain temperatures, within the bounds of the acceptable level of clinical agreement (0.5°C).10 Temperature discrepancies near the ventricles support the need for improved boundary conditions between model domains. Further refinement of the biophysical model including a more detailed representation of brain hemodynamics and local metabolism, combined with more accurate experimental brain thermometry, will facilitate an improved understanding of the role of brain temperature in healthy humans and its utility as a non-chemical biomarker in brain injury. As thermal therapies are increasingly common, the use of rigorous thermal modeling, combined with subject-specific input data to generate brain temperature predictions, is the first step towards a personalized approach for developing brain thermal biomarkers of injury, recovery, and treatment monitoring.Conclusions
In this study, a brain temperature model was developed using the first principles of underlying physics to ensure energy and mass conservation. The model was applied to simulate brain thermodynamics based on experimentally measured vessel and tissue structure. Subject-specific brain temperature predictions were compared to the experimentally measured brain temperatures acquired with CST. With the average difference <0.5°C, these results set the foundation for further work including improvement in local predictions with model refinement and further comparison with broader and more diverse experimental brain temperature datasets.Acknowledgements
MR experiments were performed at the Emory Center for Systems Imaging Core (CSIC).References
1. Sukstanskii, A.L. and Yablonskiy, D.A., Theoretical model of temperature regulation in the brain during changes in functional activity. Proc Natl Acad Sci. 2006;103(32):12144-12149.
2. Blowers, S., et al., How does blood regulate cerebral temperatures during hypothermia? Sci Rep. 2018;8(1):7877.
3. Ashburner, J. and Friston, K.J., Unified segmentation. Neuroimage. 2005;26(3):839-851.
4. Fen L., et al., neuTube 1.0: A new design for efficient neuron reconstruction software based on the swc format, Eneuro. 2015;2(1):e0049-14.
5. LaValle, S.M. and Kuffner Jr, J.J., Randomized kinodynamic planning. Int J Rob Res. 2001;20(5):378-400.
6. Maudsley, A.A., et al. Mapping of brain metabolite distributions by volumetric proton MR spectroscopic imaging (MRSI). Magn Reson Med. 2009;61:548–559.
7. Sabati, M., et al. Multivendor implementation and comparison of volumetric whole-brain echo-planar MR spectroscopic imaging. Magn Reson Med. 2015;74:1209–1220.
8. Prakash, K.N., et al. Echo planar spectroscopic imaging based temperature calibration at 7T and 3T for whole brain temperature measurement in rodents and humans. In Proceedings of the 22nd Annual Meeting of ISMRM, Milan, Italy, 2014
9. Ekwall E.M., et al. Determination of the most effective cooling temperature for the prevention of chemotherapy‑induced alopecia. Mol Clin Oncol. 2013;1(6):1065-71.
10. Suleman M.I., et al. Insufficiency in a new temporal-artery thermometer for adult and pediatric patients. Anesth. Anal. 2002;95(1):67-71.
Figures