4143
ENHANCED FPI BASED FORCE SENSOR DESIGNED FOR MRI COMPATIBLE BIOPSY NEEDLE1Institute of Biomedical Engineering, Bogazici University, Istanbul, Turkey
Synopsis
In this work, a novel design fiber optic force sensor with increased resolution thru Titanium coating is fabricated using Fabry-Pérot Interferometry (FPI) method and integrated into a biopsy needle to be used in interventional biopsy operations under MRI. The FPI based force sensor with improved resolution is tested via benchtop experiments after integrating into a MRI compatible biopsy needle. Sensor’s penetration detection, tissue characterization and differentiation capabilities are tested using different types of tissues. Overall system performance is tested via in vitro experiments under MRI using a commercial prostate phantom with leisons.
INTRODUCTION
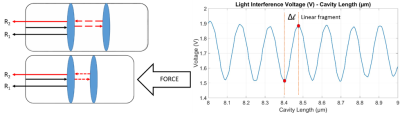
Real time needle tip force sensing capability provides an important feedback to improve both accuracy and safety of image guided minimally invasive operations1-4. Performing biopsy operations under MRI improves the overall operation process thanks to MRI’s excellent soft tissue contrast1-4, ensuring better needle targeting, predicting and avoiding potential needle deflections could be improved by a force sensor integrated to the biopsy needle. Fabry-Pérot Interferometry (FPI) based fiber optic sensors are very convenient to be integrated inside of biopsy needles and used under MRI by being small, compact, safe for clinical use and MRI compatible5. FPI based force sensors form a correlative relationship between the applied force and the back reflected light intensity. FPI sensor contains two mirror surfaces with an air cavity between them, forming a light interference pattern by creating a path difference between two reflected light beams. Light intensity of the interference pattern changes with respect to the air cavity length which depends on the applied axial force to the sensor probe.Apart from accurate needle targeting, force sensing capabilities of biopsy needles can be used for tissue characterization. Different tissue types can be differentiated using their different mechanical characteristics such as stiffness. Therefore, Biopsy needles with integrated force sensors have a great potential for cancer diagnosis by detecting tissue types with different stiffnesses such as healthy tissues, benign or malignant tumorous tissues6-8. A sufficient sensor resolution has to be achieved for detecting small differences in stiffness and hence being successful in tissue differentiation.
METHODS
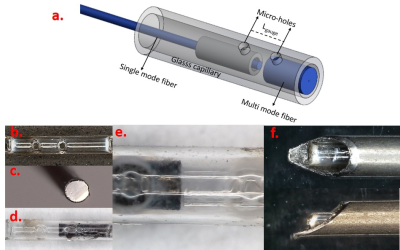
Two mirror surfaces that is required for FPI based force sensing via reflected light beams are formed by end surfaces of a single mode optical fiber and a larger diameter multimode optical fiber fixed inside of a glass capillary. Two micro-holes are formed on the glass capillary as fixing points for optical fibers. The distance between the fixing points of optical fibers is defined as the gauge length (Lgauge) which is an important parameter for determining the force range of the sensor with respect to the equation below9-11.ΔLcavity=F(1-2µ)Lgauge/(Eπ (ro2-ri2)) Lcavity: Cavity Length E:Elastic modulus F: Applied force ro: outer diameter µ: Poissons ratio ri: inner diameter Lgauge: Gauge length
Using a multimode fiber with a larger reflective surface as the secondary optical fiber helps obtaining the maximum possible SNR but it does not increase the signal intensity. A high reflectance mirror surface is created by Titanium coating for the multimode fiber. This way increased sensor resolution can be achieved with increased reflected light intensity. Sensor fabrication steps respectively; glass capillary fabrication with micro-holes (fig.2.b), Ti coated multimode fiber (fig.2.c), fixing optical fibers into the glass capillary through micro-holes (fig.2.d-e), Integrating the sensor probe to a biopsy needle (fig.2.e), are shown in figure 2.
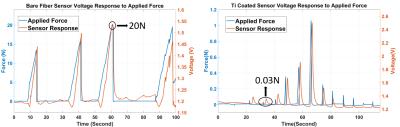
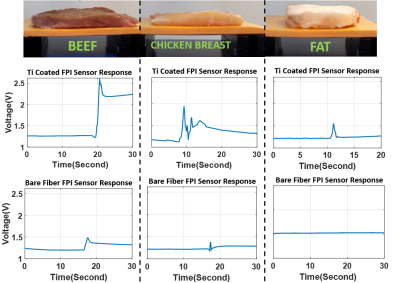
The performance of the biopsy needle, integrated with FPI based force sensor is tested thru benchtop experiments. Sensor resolution and the measurable force range is determined by comparing fabricated sensor response and a commercial force sensor (LF-Plus, Lloyd Instruments Ltd) against an applied axial force. Sensor’s capability of penetration detection and tissue differentiation is tested using different tissue types such as beef, chicken breast, fat tissue. Experiments under MRI are performed to test sensor’s MRI compatibility and its response during penetration into a commercial prostate phantom with lesions (CIRS Model-070L).
RESULTS
It is shown in figure 3 that FPI based force sensor successfully detects the applied force and responds to the changes accurately. The maximum measurable force is found to be 20 N. By coating the multimode fiber with Titanium and increasing the SNR of the sensor, the resolution of the sensor is found to be 0.03N.Sensor probes, designed with bare multimode fiber and Ti coated multimode fiber, are both tested using different tissue types and their response during penetration into tissues are compared in figure 4. It is seen that Ti coated sensor probe has a higher SNR and a higher resolution.
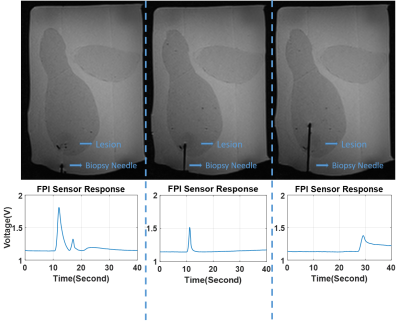
The sensor response was recorded during needle penetration into a commercial prostate phantom with lesions under MRI. Figure 5 shows that custom designed biopsy needle does not generate any image degradation. It can be seen that needle penetration in three steps, through the skin, into the tumorous lesion and out of the tumorous lesion respectively, of the prostate phantom can be detected with custom designed sensor.
DISCUSSION AND CONCLUSION
Using custom designed biopsy needles with force measurement capability under MRI, the accuracy and safety of image guided minimally invasive procedures especially biopsies, can be improved. Improving our previous design[12] by Ti coating on the reflective surface of the multimode fiber results in a higher resolution. As shown through in vitro experiments, high resolution force sensing biopsy needles can detect penetration and it can differentiate tissues with different stiffness. The response of the sensor differs when it is penetrating into beef, chicken breast or fat tissue. Regarding the tissue differentiation and stiffness detection capabilities; biopsy needles with high resolution force sensing capability have a great potential to be used for cancer diagnosis without needing a biopsy operation at all since mechanical characteristics of malignant and benign tumorous tissues or healthy tissues varies.Acknowledgements
No acknowledgement found.References
1. D. A. Woodrum, K. R. Gorny, B. Greenwood, and L. A. Mynderse, “MRI-Guided Prostate Biopsy of Native and Recurrent Prostate Cancer,” Semin. Intervent. Radiol. 33(3), 196–205 (2016).
2. I. M. Noebauer-Huhmann, M. A. Weber, R. K. Lalam, S. Trattnig, K. Bohndorf, F. Vanhoenacker, A. Tagliafico C. van Rijswijk, J. C. Vilanova, P. D. Afonso, M. Breitenseher, I. Beggs, P. Robinson, M. C. de Jonge, C. Krestan, and J. L. Bloem, “Soft tissue tumors in adults: ESSR-approved guidelines for diagnostic imaging,” Semin. Musculoskelet. Radiol 19(5), 475–482 (2015).
3. M. Roethke, A. G. Anastasiadis, M. Lichy, M. Werner, P. Wagner, S. Kruck, C. D. Claussen, A. Stenzl, H. P. Schlemmer, and D. Schilling, “MRI-guided prostate biopsy detects clinically significant cancer: analysis of a cohort of 100 patients after previous negative TRUS biopsy,” World J. Urol. 30(2), 213–218 (2012).
4. Lindenberg, L., Ahlman, M., Turkbey, B., Mena, E., & Choyke, P. “Evaluation of prostate cancer with PET/MRI,” J. Nucl. Med. 57(3), 111-116 (2016).
5. H. Su, I. I. Iordachita, J. Tokuda, N. Hata, X. Liu, R. Seifabadi, S. Xu, B. Wood, and G. S. Fischer, “Fiber Optic Force Sensors for MRI-Guided Interventions and Rehabilitation: A Review,” IEEE Sens. J. 17(7), 1952–1963 (2017).
6. Ahmad, S., Cao, R., Varghese, T., Bidaut, L., & Nabi, G. (2013). Transrectal quantitative shear wave elastography in the detection and characterisation of prostate cancer. Surgical endoscopy, 27(9), 3280-3287.
7. Krouskop, T. A., Wheeler, T. M., Kallel, F., Garra, B. S., & Hall, T. (1998). Elastic moduli of breast and prostate tissues under compression. Ultrasonic imaging, 20(4), 260-274.
8. Bayat, M., Denis, M., Gregory, A., Mehrmohammadi, M., Kumar, V., Meixner, D., Fazzio, R. T., Fatemi, M., and Alizad, A., \Diagnostic features of quantitative comb-push shear elastography for breast lesion differentiation," PloS one 12(3), e0172801 (2017).
9. K. Bremer, E. Lewis, G. Leen, B. Moss, S. Lochmann, I. Mueller, and J. Schrotter, “Fibre optic pressure and temperature sensor for geothermal wells,” Sensors (Basel) 2010, 538–541 (2010).
10. X. Zhou, Q. Yu, W. Peng, B. Moss, S. Lochmann, I. Mueller, and J. Schrotter, “Simultaneous measurement of down-hole pressure and distributed temperature with a single fiber,” Meas. Sci. Technol. 23(8), 85–102 (2012).
11. A. Wang, H. Xiao, J. Wang, Z. Wang, W. Zhao, and R. G. May, “Self-calibrated interferometric-intensity-based optical fiber sensors,” J. Lightwave Technol. 19(10), 1445 (2001).
12. Ulgen, N. O., Uzun, D., & Kocaturk, O. (2019). Phantom study of a fiber optic force sensor design for biopsy needles under MRI. Biomedical optics express, 10(1), 242-251.