4139
MRI-Guided Cryoablation and Image-Guided Immunotherapy in Naturally Occurring Canine Osteosarcoma
Dara Kraitchman1, Cory Brayton1, Rebecca Krimins1, Byung-Hak Kang1, Zachary Millman1, Edward McCarthy1, and Brian Ladle1
1Johns Hopkins University School of Medicine, Baltimore, MD, United States
1Johns Hopkins University School of Medicine, Baltimore, MD, United States
Synopsis
Improvements in outcomes with current chemotherapy and surgery treatment approaches for patients with osteosarcoma have plateaued over the past three decades necessitating new treatment paradigms. A novel therapy for osteosarcoma of MR-guided cryotherapy in conjunction with intratumoral injection of an immune adjuvant in the clinically relevant animal model of spontaneously occurring osteosarcoma in client-owned dogs with the intent of priming an adaptive immune response capable of delaying or preventing the occurrence of metastatic disease is demonstrated.
Introduction
Osteosarcoma (OSA) occurs in both children/young adults and dogs with an annual incidence of 4501 and 25,000 cases, respectively, making dogs a useful comparative oncology model for the development of OSA treatments.2 OSA metastasizes in about 30-40% of children making the cancer incurable, and there has been little progress in developing new treatments for metastatic OSA in the past 30 years.3 Canine OSA has many striking similarities with respect to genetics and tumor biology, with metastatic spread more likely to occur than in pediatric OSA.4 Thus, new treatment approaches beyond standard of care (SOC) surgery and chemotherapy are sorely needed. Recent advances in immunotherapy have not shown activity in OSA patients reflecting the poor pre-existing immune activation in OSA.Cryotherapy, which can directly kill cells in tumor has also shown the ability to activate the patient’s own immune system, i.e., eradicate tumor cells far from the site of where the primary tumor was frozen. Using Magnetic Resonance Imaging (MRI), one can precisely determine the extent of tumor tissue that is frozen by cryotherapy to accurately kill tumor cells without exposure to ionizing radiation. In the current study, we seek to develop MR-guided cryotherapy in dogs in conjunction with image-guided intratumoral injection of an immune adjuvant in naturally occurring OSA in dogs and study the native immune response.
Methods
All canine studies were approved by the institutional animal care and use committee. Dogs of either sex and any breed with radiographic criteria and histopathology suggestive of appendicular OSA were recruited from referring veterinarians to the Center for Image-Guided Animal Therapy at Johns Hopkins University. Informed consent was obtained from all dog owners. Dogs were randomized to received cryotherapy alone (Cryo), intratumoral immunotherapy (STING), or both cryotherapy/immunotherapy (Combo).Complete blood counts, blood chemistries, and chest films were acquired to assess anesthetic risk. After placement of an IV catheter, the dogs were anesthetized and placed on isoflurane anesthesia and mechanical ventilation. A chest cone beam CT (DynaCT Body preset, Axiom Artis Zee, Siemens) was acquired to assess the presence of pulmonary metastases. For MR-guided cryotherapy, the dogs were moved to a 1.5T wide-bore MRI scanner (Espree, Siemens) using a transfer table for imaging of the affected limb. After obtaining scout images, proton density (PD) images (5800 ms TR; 26 ms TE; 4 NSA; 18 ETL; 151 Hz/pixel BW; 4 mm slice thickness; 448x436 matrix; and 270 mm FOV) were acquired in the axial and sagittal planes for treatment planning. Five contiguous axial images were then planned for the cryoablation needle path with the skin entry point marked by MR-visible fiducials. A single loop coil was centered on the planned skin entry point. The skin was then sterilely prepared and draped, and a local anesthetic block was performed. Using an MR-conditional 4mm serrated drill, co-axial trocar sheath, and blunt ejector (In Vivo), the trocar-sheath was advanced into the osseous lesion using intermittent metal-artifact reducing, TSE MRI axial images (1940 ms TR; 23 ms TE; 1 NSA; 24 ETL, 5 mm slice thickness, 280x280 FOV; 384x384 image matrix, and 407 Hz/pixel BW). Once the near cortex of the tumor was penetrated, the sheath was locked and the stylet was replaced with the bone drill to obtain biopsy specimens. After the biopsy specimens were obtained, an MR-compatible cryoablation needle (IceSeed, Galil) was placed and two 10-minute freeze/5-minute thaw cycles were performed using an MR-compatible cryoablation system (Galil) while the TSE MRI was repeated to document the extent of the ice ball. The cryoablation needle and sheath were then removed and PD MRI images in the axial and sagittal planes were repeated prior to recovery of the dog.
For the STING or Combo arms, 100 μl of an immunotherapy was injected intratumorally under MR or X-ray guidance and repeated one week later. After treatment, all dogs received non-steroidal analgesics and oral antibiotics until amputation at ~2 weeks post-cryoablation. Histopathology was performed on the amputated limb and peripheral blood was collected at amputation to assess the tumor infiltrating lymphocyte response with CT at 3 & 6-months post-treatment.
Results
Seven dogs have been enrolled without evidence of pulmonary metastases and randomized to Cryo (n=2), STING (n=3), or Combo (n=1). One dog was excluded for non-neoplastic biopsy. MR-or CT-guided bone biopsies confirmed OSA (n=3), chondrogenic OSA (n=1), chondrosarcoma (n=1), and sarcoma (n=1). The tumor size in all dogs exceeded the maximum iceball dimension from a single needle freeze-thaw. Thus, no tumor was completely ablated in the study. Anatomical pathology revealed a necrotic zone that was concordant with the iceball location and size observed on MRI (Figure 1). Regardless of tumor type, an inflammatory response was seen in response to cryoablation or immunotherapy (Figure 2). Only one dog (in STING arm) received chemotherapy after amputation. Mean survival time (MST) was longer in the STING than Cryo arms (Table 1).Conclusions
MR-guided biopsy/cryoablation can be successfully performed with accurate monitoring of the tumor dimensions in the bone to avoid critical vascular and neurological structures. While an inflammatory response to cryotherapy was evoked, improved MST in the immunotherapy arm suggests that cryoablation alone may be insufficient to invoke an immunotherapeutic response to the tumor antigens.Acknowledgements
We would like to thank the Children's Cancer Foundation for funding and Inez Vasquez, Jessica Lawyer, and Maggie Phillips with assistance in the canine studies.References
- Mirabello L, Troisi RJ and Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: data from the Surveillance, Epidemiology, and End Results Program. Cancer. 2009;115:1531-43.
- Withrow SJ, Powers BE, Straw RC and Wilkins RM. Comparative aspects of osteosarcoma. Dog versus man. Clin Orthop Relat Res. 1991:159-68.
- Varan A, Yazici N, Aksoy C, Gedikoglu G, Yalcin B, Akyuz C, Kutluk T and Buyukpamukcu M. Treatment results of pediatric osteosarcoma: twenty-year experience. J Pediatr Orthop. 2007;27:241-6.
- Paoloni M, Davis S, Lana S, Withrow S, Sangiorgi L, Picci P, Hewitt S, Triche T, Meltzer P and Khanna C. Canine tumor cross-species genomics uncovers targets linked to osteosarcoma progression. BMC Genomics. 2009;10:625 https://www.ncbi.nlm.nih.gov/pubmed/20028558.
Figures
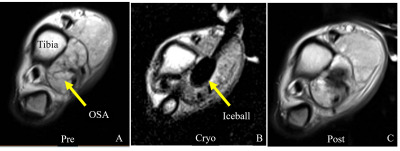
Figure 1: (A) Axial PD image of the distal dog
tibia affected with osteosarcoma (OSA). (B) Image of the iceball targeted to
the tumor. (C) MRI acquired shortly after treatment.
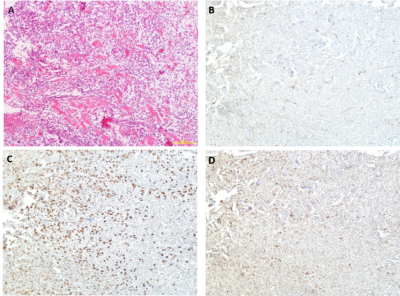
Figure 2: Histological Staining of Cryoablated Limb in a Dog with Osteosarcoma.
(A) Hematoxylin and eosin (H&E) staining in area adjacent to necrotic tissue from cryoablation. (B)
CD31 immunostaining (brown DAB enhancement) for blood vessels shows relatively
normal appearance. (C) IBA-1 immunostaining (brown DAB enhancement) for
activated macrophages demonstrates a robust infiltrate. (D) CD3 immunostaining
(brown DAB enhancement) for mature T cells demonstrates a moderate response.
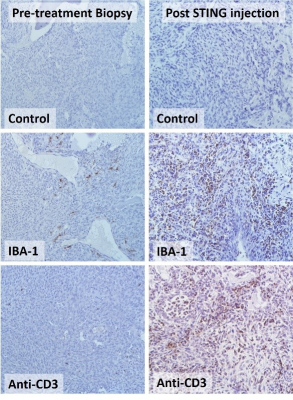
Figure 3: Histological
Staining of pre-treatment (left panels) and post STING agonist-treated (right
panels) canine OSA. Control (secondary antibody alone), IBA-1
immunostaining (brown DAB enhancement) for activated macrophages, and anti-CD3
immunostaining (brown DAB enhancement) for mature T cells indicates a robust
immune infiltrate after intratumoral STING agonist treatment.
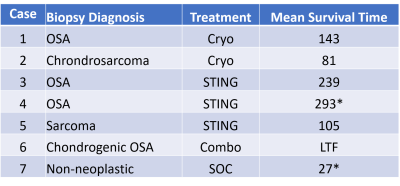
Table 1: MR- or CT-guided bone biopsy diagnosis with treatment received and mean survival time after diagnosis. Dogs that are still alive are denoted with an asterisk. (LTF is lost to follow-up after 60 days.)