4136
A feasibility study of Magnetic Resonance Fingerprinting for multi-contrast temperature mapping in both aqueous and adipose tissues
Megan E Poorman1,2, Rasim Boyacioglu3, William A Grissom4, Mark A Griswold3, and Kathryn E Keenan1
1Physical Measurement Laboratory, National Institute of Standards and Technology, Boulder, CO, United States, 2Department of Physics, University of Colorado Boulder, Boulder, CO, United States, 3Department of Radiology, Case Western Reserve Univeristy, Cleveland, OH, United States, 4Vanderbilt University Institute of Imaging Science, Nashville, TN, United States
1Physical Measurement Laboratory, National Institute of Standards and Technology, Boulder, CO, United States, 2Department of Physics, University of Colorado Boulder, Boulder, CO, United States, 3Department of Radiology, Case Western Reserve Univeristy, Cleveland, OH, United States, 4Vanderbilt University Institute of Imaging Science, Nashville, TN, United States
Synopsis
Temperature monitoring in both adipose and aqueous tissues is important for guidance of thermal therapies in vivo. However, the proton resonant frequency shift for thermometry is only reliable in aqueous tissues. Temperature mapping in adipose has been explored using relaxation, but is limited by the accuracy and speed of the method used. MR Fingerprinting provides a framework for mapping differences due to multiple tissue properties simultaneously. This work explores adapting the MRF framework to allow temperature changes to be mapped directly in all tissue types, and simulates a dictionary update method that could offer improved temporal resolution over standard MRF.
Introduction
Temperature mapping in both adipose and aqueous tissue is important for guidance of thermal therapy in vivo. The proton resonant frequency-shift (PRFS)1 is commonly used to map temperature changes in aqueous tissues, but is unreliable in adipose tissue. To overcome this barrier, some techniques rely on changes in T1 or T2 to monitor changes in temperature in adipose tissue2. However, these methods require accurate and precise mapping of T1 and T2, which can be difficult to achieve in the high temporal resolution (~3s) required for guidance of therapies. In this work, we propose using Magnetic Resonance Fingerprinting (MRF)3 adapted for mapping of temperature. MRF is a quantitative imaging method that is capable of mapping multiple tissue properties simultaneously. However, conventional MRF acquisitions are too slow to allow real-time monitoring (~40s). In this feasibility study, we explore using an accelerated quadratic RF MRF technique (MRFqRF)4 to map temperature derived from changes in T1, T2, and B0 in both aqueous and adipose phantoms. To overcome temporal resolution constraints and better inform sequence design, we propose an evolving temperature dictionary reconstruction method and explore its accuracy over a range of image SNRs and heating levels.Methods
Data AcquisitionA phantom (beef muscle or pork fat) was placed within a water bath and in a 20-channel head coil at isocenter of the MRI scanner. A laser ablation system (PhotoMedex, LaserPro980) was used to locally heat the phantom via ablation probe placed within the tissue. Fiber optic temperature probes (Luxtron, LumaSense Technologies) were placed at the laser focal point and a distal site. An axial slice was chosen that encompassed both the ablation probe and fiber optic probe tips to map temperature in the hotspot. Baseline MRF data was acquired prior to heating with the laser for 48s (1.5W CW). MRF data was acquired throughout heating and during subsequent cooling to room temperature.
Pulse Sequence
MRFqRF was implemented on a 3T MRI scanner (Skyra, Siemens) with 876 timepoints (Figure 1A, TR/TE = 11/2.2ms). The off-resonance frequency was swept 2.88Hz/TR by applying quadratic phase to the RF pulses. This acquisition increases sensitivity of MRFqRF to B0 changes5. To increase T1 sensitivity, inversion pulses were applied after every 219 timepoints. This block of 876 timepoints was repeated continuously throughout the experiment.
Conventional MRFqRF Reconstruction
Acquired MRFqRF data was reconstructed with randomized SVD and matched to a dictionary constructed over a range of low resolution T1/T2/B0 values (min:max 10:3000/2:500/-50:50). Each block’s 876 timepoints was matched separately to the dictionary to reconstruct T1, T2, and B0 maps (Figure 1A). Quadratic interpolation of MRFqRF tissue property maps achieved fine property resolution.
Improved Temperature Reconstruction
To improve the temporal resolution and sensitivity of the MRFqRF measurements, an evolving temperature dictionary was created (Figure 1B). This dictionary consists of two concatenated segments: the first segment is the baseline signal evolution (876 timepoints) with the matched T1, T2, and B0 values. The second segment is a shortened dynamic consisting of 219 timepoints (2.6s) from the next MRF block, acquired during temperature change. This shortened dynamic is fit to a temperature dictionary generated over a feasible range of temperature changes (0°C to 40°C). This dictionary can be generated on the fly and used to fit each subsequent dynamic. The accuracy and sensitivity of this method was explored in simulation over a range of SNRs and temperature changes.
Results and Discussion
Changes in T1, T2, and B0 generated from conventional MRFqRF reconstruction can be seen in Figure 2. In the muscle phantom, there is a change in resonant frequency with temperature, coinciding with the laser output. However, the temporal resolution is insufficient to fully capture the heating pattern. The adipose phantom exhibits no such frequency shift with temperature, but T2 increases with the laser output. In both phantoms the T1 and T2 reconstructions have large variations through time due to noise, impeding the accuracy of this reconstruction.A representative temperature dictionary from the proposed temperature reconstruction is shown in Figure 3. The effect of B0 shifts in water and T1/T2 changes in fat are visible in the dictionary as shifts in peak locations or changes in peak magnitudes, respectively. The dictionary was generated in 0.41s on a 16GB RAM computer and matching took 0.03s, implying that this method is feasible for online temperature reconstruction.
Results from the simulated heating experiment with temperature dictionary reconstruction are shown in Figure 4. For both muscle and fat, SNR of 1 is too low to accurately reconstruct temperature. An SNR of 2.5 enables the method to imprecisely track dynamic changes, while an SNR of 10 yields a precise and accurate reconstruction.
The sensitivity of this temperature reconstruction method to a 1°C change in temperature is shown in Figure 5. An SNR of >2.5 is needed to detect this level of change, which is feasible with MRFqRF acquisitions.
Conclusions
We explored MRF for multi-contrast temperature mapping in multiple tissue types. The conventional MRF T1, T2, and B0 maps show changes with temperature in both tissue types, but are limited by temporal resolution and SNR. The proposed temperature reconstruction has potential to overcome these limitations, suggesting that a fast and accurate MRF thermometry method is feasible.Acknowledgements
This work was supported by Siemens Healthcare and a cooperative grant between the University of Colorado and NIST.References
- Ishihara Y, Calderon A, et al. A precise and fast temperature mapping use water proton chemical shift. Magnetic Resonance in Medicine 1995 34:814-823
- Odéen H and Parker D. Non-Invasive Thermometry with Magnetic Resonance Imaging. Theory and Applications of Heat Transfer in Humans, Volume 1, 2018
- Ma D, Gulani V, et al. Magnetic resonance fingerprinting. Nature 2013 495:187-192
- Wang C, Coppo S, Mehta B, et al. Magnetic resonance fingerprinting with quadratic RF phase for measurement of T2* simultaneously with δf ,T1, and T2. Magnetic Resonance in Medicine 2019 81(3):1849-1862.
- Magnetic Resonance Fingerprinting with Quadratic RF Phase for Continuous Temperature Monitoring in Aqueous Tissues. Submitted to ISMRM 2020
Figures
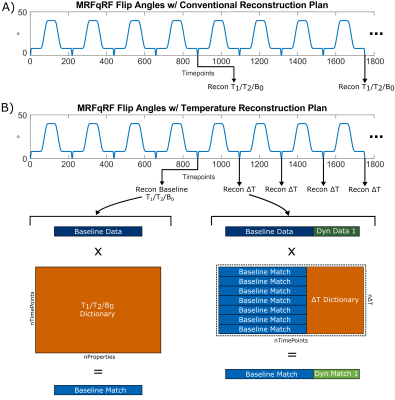
Figure 1: (A) Conventional MRFqRF
reconstruction. The block of 876 timepoints was repeated dynamically and fit to
a dictionary, generating T1, T2, and B0 maps after each block that can be
related to temperature changes. (B) Temperature reconstruction plan. First a
block of 876 timepoints is matched to obtain baseline T1, T2, and B0 values.
Subsequent flip angle sweeps are split into blocks of 219 timepoints, termed
dynamics, and matched to a concatenated temperature dictionary.
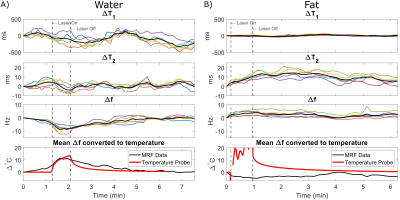
Figure 2:
Changes in tissue properties with temperature, obtained from maps generated
with the conventional MRF reconstruction method. Individual voxels are
displayed in color and their mean value is displayed in black. Frequency shifts
(Δf) in water (A) correspond to heating with the
laser. However, temporal resolution was insufficient to capture the full
dynamic changes compared to the temperature probe. T2 rises in fat (B), but no
change in frequency. The low SNR of the conventional reconstruction confounds
the temperature measurement.
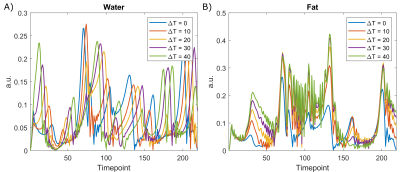
Figure 3: Sample temperature update
dictionary entries for a given 219 timepoint dynamic, in both water (A) and fat
(B). The effect of B0 shifts due to temperature can be seen in the water
dictionary as a shift in the location of the peaks. Since fat changes are
dominated by T1 and T2 effects rather than B0, the peaks change in magnitude,
rather than shift in time with temperature.
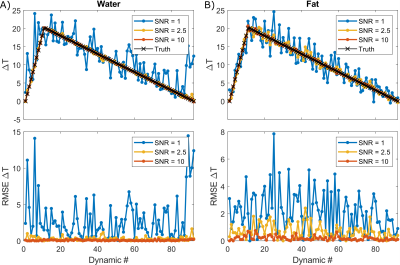
Figure 4: Simulated
temperature evolution solved with proposed reconstruction method in water (A)
and fat (B) at varied SNR levels. To achieve an RMSE <1 °C at all time points, an SNR > 2.5 is needed.
Noted that the acceptable SNR cutoffs may shift depending on tissue type. The
average RMSE in water and fat, respectively was 2.95°C and 2.12°C
for SNR = 1, 0.28°C
and 0.68°C for SNR = 2.5, and 0.08°C and 0.19°C for
SNR = 10.
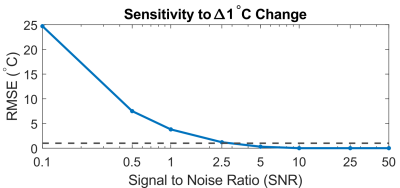
Figure 5: Sensitivity
of the temperature reconstruction plan with respect to a 1°C change in temperature. An SNR of > 2.5 is required to achieve this level of
detection, which is feasible with an MRFqRF acquisition.